| CPC C08G 65/338 (2013.01) [C08G 73/1007 (2013.01); C08G 73/1039 (2013.01); C08G 73/1042 (2013.01); C08G 73/1064 (2013.01); C08G 73/1067 (2013.01); C08L 71/00 (2013.01); C08L 79/08 (2013.01); C08G 65/4012 (2013.01)] | 10 Claims |
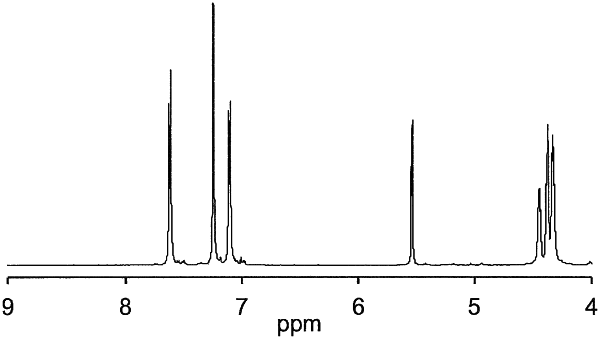
|
1. A composition comprising an electron-donating polymer (D) and an electron-withdrawing polymer (A), wherein
the electron-donating polymer (D) has a constitutional unit represented by the formula (1a):
*—X1a—O—Y1a—O—* (1a)
wherein X1a is a divalent group represented by the formula (2a) or the formula (2b):
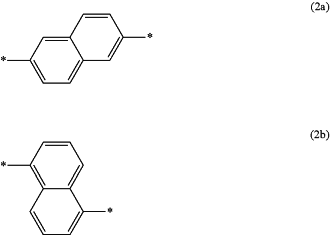 wherein * is a bonding position,
Y1a is a divalent group represented by the formula (3a):
 wherein * is a bonding position, and
* is a bonding position, and
the electron-withdrawing polymer (A) has a constitutional unit represented by formula (4a):
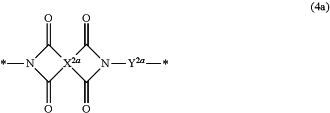 wherein X2a is a tetravalent group represented by any of the formula (5a) to the formula (5c):
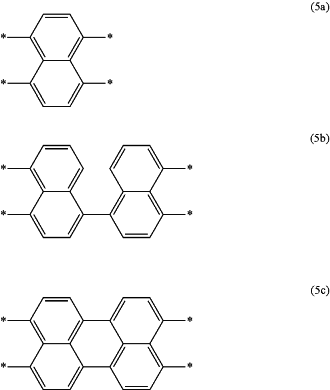 wherein * is a bonding position,
Y2a is a divalent group represented by any of the formula (6a) to the formula (9a):
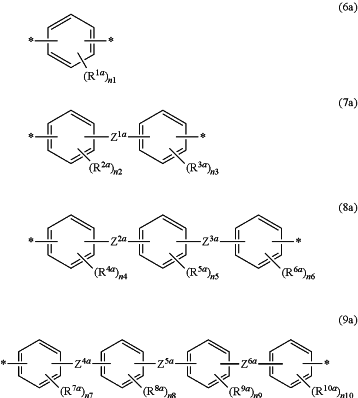 wherein n1 is an integer of 1-4,
n2-n10 are each independently an integer of 0-4,
R1a-R10a are each independently a C1-10 alkyl group optionally substituted by a halogen atom, a C1-10 alkoxy group optionally substituted by a halogen atom, a hydroxy group, a halogen atom, a nitro group, a formyl group, a cyano group, a sulfo group, a phenyl group optionally substituted by W1a, a thienyl group optionally substituted by W1a, or a furyl group optionally substituted by W1a,
W1a is a C1-10 alkyl group optionally substituted by a halogen atom, a C1-10 alkoxy group optionally substituted by a halogen atom, a hydroxy group, a halogen atom, a nitro group, a formyl group, a cyano group, or a sulfo group,
when n1 to n10 are each an integer of 2 to 4, plural R1a to R10a are optionally the same as or different from each other,
at least one of R1a in the number of n1 is a sulfo group,
at least one selected from the group consisting of R2a in the number of n2 and R3a in the number of n3 is a sulfo group,
at least one selected from the group consisting of R4a in the number of n4, R5a in the number of n5 and R6a in the number of n6 is a sulfo group,
at least one selected from the group consisting of R7a in the number of n7, R8a in the number of n8, R9a in the number of n9 and R10a in the number of n10 is a sulfo group,
Z1a-Z6a are each independently a single bond, a C1-2 alkylene group optionally substituted by a halogen atom, a C3-10 alkylene group, sulfonyl group, a carbonyl group, *—CONH—*, *—NHCO—*, *—C(R11a)(R12a)—*, or an oxy group,
R11a and R12a are each independently a C1-3 alkyl group optionally substituted by a halogen atom, or R11a and R12a are bonded to each other to form a C3-6 hydrocarbon ring together with a carbon atom bonded thereto, and
* is a bonding position, and
* is a bonding position.
|