| CPC C07D 495/20 (2013.01) [C07D 519/00 (2013.01)] | 55 Claims |
|
1. A compound represented by the following structural formula:
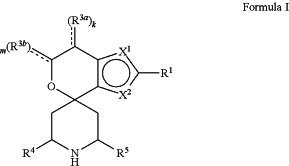 a tautomer thereof, a deuterated derivative of that compound or tautomer, or a pharmaceutically acceptable salt of any of the foregoing, wherein:
X1 is selected from S and —CR2a and X2 is selected from S and —CR2b, wherein:
one of X1 and X2 is S;
when X1 is S, then X2 is —CR2b; and
when X2 is S, then X1 is —CR2a;
R1 is selected from hydrogen, halogen, cyano, —OH, C1-C6 alkyl, C1-C6 alkoxy, C3-C6 cycloalkyl, and phenyl, wherein:
the C1-C6 alkyl of R1 is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, —OH, —NH2, —NH(C1-C4 alkyl), —N(C1-C4 alkyl)2, and C1-C4 alkoxy;
the C1-C6 alkoxy of R1 is optionally substituted with 1 to 3 groups independently selected from halogen;
the C3-C6 cycloalkyl of R1 is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, —OH, —NH2, —NH(C1-C4 alkyl), —N(C1-C4 alkyl)2, C1-C4 alkyl, C1-C4 alkoxy, —C(═O)NH2, —C(═O)NH(C1-C4 alkyl), and —C(═O)N(C1-C4 alkyl)2; and
the phenyl of R1 is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, —OH, —NH2, —NH(C1-C4 alkyl), —N(C1-C4 alkyl)2, C1-C4 alkyl, C1-C4 alkoxy, —C(═O)NH2, —C(═O)NH(C1-C4 alkyl), and —C(═O)N(C1-C4 alkyl)2;
R2a is selected from hydrogen, halogen, cyano, —OH, ═O, and C1-C6 alkyl, wherein:
the C1-C6 alkyl of R2a is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, —OH, and C1-C4 alkoxy;
R2b is selected from hydrogen, halogen, cyano, —OH, ═O, and C1-C6 alkyl;
R3a is selected from halogen, cyano, —OH, C1-C6 alkyl, and ═O; wherein:
the C1-C6 alkyl of R3a is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, and —OH;
R3b is selected from C1-C2 alkyl and ═O; wherein:
the C1-C2 alkyl of R3b is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, and —OH;
 , for each occurrence, is a single bond when R3a is selected from halogen, cyano, —OH, C1-C6 alkyl or when R3b is selected from C1-C2 alkyl; or alternatively , for each occurrence, is a single bond when R3a is selected from halogen, cyano, —OH, C1-C6 alkyl or when R3b is selected from C1-C2 alkyl; or alternatively  , for each occurrence, is a double bond when R3a is ═O or when R3b is ═O; , for each occurrence, is a double bond when R3a is ═O or when R3b is ═O;R4 is selected from C1-C6 alkyl, —C(═O)O(C1-C4 alkyl), C2-C6 alkynyl, and
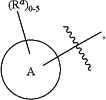 wherein:
the C1-C6 alkyl of R4 is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, —OH, —NH2, —NH(C1-C4 alkyl), —N(C1-C4 alkyl)2, C1-C4 alkoxy, —C(═O)NH2, —C(═O)NH(C1-C4 alkyl), —C(═O)N(C1-C4 alkyl)2, C3-C6 cycloalkyl, 5 to 10-membered heterocyclyl, phenyl, and 5 to 10-membered heteroaryl;
Ring A is selected from C3-C12 carbocyclyl, 3 to 12-membered heterocyclyl, C6 and C10 aryl, and 5 to 10-membered heteroaryl, wherein Ring A is optionally substituted with 1, 2, 3, 4, or 5 Ra groups; wherein:
Ra, for each occurrence, is independently selected from halogen, cyano, C1-C6 alkyl, C2-C6 alkenyl, C1-C6 alkoxy, C1-C6 haloalkyl, C1-C6 haloalkenyl,
C1-C6 haloalkoxy, —C(═O)NRhRi, —NRhRi, —NRhC(═O)Rk, —NRhC(═O)ORk, —NRhC(═O)NRiRj, —NRhS(═O)pRk, —ORk, —OC(═O)Rk, —OC(═O)ORk, —OC(═O)NRhRi, —[O(CH2)q]rO(C1-C6 alkyl), —S(═O)pRk, —S(═O)pNRhRi, —C(═O)ORk, C3-C12 carbocyclyl, 3- to 12-membered heterocyclyl, C6 and C10 aryl, and 5- to 10-membered heteroaryl; wherein:
the C1-C6 alkyl, C1-C6 alkoxy, and the C2-C6 alkenyl of Ra are each optionally substituted with 1 to 3 groups independently selected from C6 to C10 aryl (optionally substituted with 1 to 3 Rm groups), 5- to 10-membered heterocyclyl (optionally substituted with 1 to 3 Rm groups), 5- to 10-membered heteroaryl (optionally substituted with 1 to 3 Rm groups), cyano, —C(═O)Rk, —C(═O)ORk, —C(═O)NRhRi, —NRhRi,
—NRhC(═O)Rk, —NRhC(═O)ORk, —NRhC(═O)NRiRj, —NRhS(═O)pRk, —ORk, —OC(═O)Rk, —OC(═O)ORk, —OC(═O)NRhRi, —S(═O)pRk, —S(═O)pNRhRi, and C3-C6 carbocyclyl (optionally substituted with 1 to 3 Rm groups);
the C3-C12 carbocyclyl, the 3 to 12-membered heterocyclyl, the C6 and C10 aryl, and the 5 to 10-membered heteroaryl of Ra are each optionally substituted with 1 to 3 groups independently selected from halogen, cyano, C1-C4 alkyl, —NRhRi, and —ORk; wherein:
Rh, Ri, and Rj, for each occurrence, are each independently selected from hydrogen, C1-C4 alkyl, C6-C10 aryl, and C3-C6 cycloalkyl; wherein:
the C1-C4 alkyl of any one of Rh, Ri, and Rj is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, and —OH;
Rk, for each occurrence, are each independently selected from hydrogen, C1-C4 alkyl, 5- to 10-membered heterocyclyl, and C3-C6 carbocyclyl; wherein:
the C1-C4 alkyl of any one of Rk is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, and —OH;
Rm, for each occurrence, is independently selected from halogen, cyano, oxo, C1-C6 alkyl, C1-C6 alkoxy, —S(═O)pRk, and —ORk; wherein:
the C1-C6 alkyl of Rm is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, and —OH;
R5 is selected from C1-C6 alkyl, —C(═O)O(C1-C4 alkyl), C3-C12 carbocyclyl, 3- to 12-membered heterocyclyl, C6 and C10 aryl, and 5- to 10-membered heteroaryl; wherein:
the C1-C6 alkyl of R5 is optionally substituted with 1 to 3 groups independently selected from halogen, cyano, —OH, —NH2, —NH(C1-C4 alkyl), —N(C1-C4 alkyl)2, C1-C4 alkoxy,
—C(═O)NH2, —C(═O)NH(C1-C4 alkyl), and —C(═O)N(C1-C4 alkyl)2;
the C3-C12 carbocyclyl, the 3 to 12-membered heterocyclyl, the C6 and C10 aryl, and the 5 to 10-membered heteroaryl of R5 are each optionally substituted with 1 to 3 groups independently selected from halogen, cyano, —OH, —NH2, —NH(C1-C4 alkyl) (optionally substituted with —OH), —N(C1-C4 alkyl)2, C1-C5 alkyl (optionally substituted with —OH), C1-C4 alkoxy, —C(═O)NH2, —C(═O)NH(C1-C4 alkyl), —NHC(═O)(C1-C4 alkyl),
—C(═O)(C1-C4 alkoxy), and —C(═O)N(C1-C4 alkyl)2;
k is an integer selected from 0, 1, and 2, wherein:
when R3a is selected from halogen, cyano, —OH, and C1-C6 alkyl, k is 1 or 2; and
when R3a is ═O, k is 1;
m is an integer selected from 0, 1, and 2, wherein:
when R3b is selected from C1-C2 alkyl, m is 1 or 2; and
when R3b is ═O, m is 1;
p is an integer selected from 1 and 2; and
q and r are each an integer selected from 1, 2, 3, and 4.
|