| CPC C07D 493/08 (2013.01) [C07B 57/00 (2013.01); C07B 2200/07 (2013.01)] | 8 Claims |
|
1. A method for separating an optically active hydroxy cineole derivative of formula (I-R),
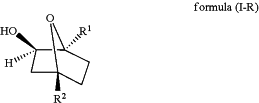 or an optically active hydroxy cineole derivative of formula (I-S),
 wherein
R1 is methyl and
R2 is isopropyl;
from a mixture comprising the enantiomers of formula (I-R) and formula (I-S), the method comprising the steps of:
i) Providing a suspension comprising a mixture of the enantiomers of formula (I-R) and formula (I-S), wherein the desired enantiomer is present in the mixture in an amount of ≥51 to ≤95 wt.-%, related to the sum of the enantiomers of the mixture, in at least one non-polar solvent;
ii) Separating the desired enantiomer by lixiviation consisting of stirring the suspension obtained in step (i) at temperature in the range of ≥10° C. to reflux temperature of the non-polar solvent such that the desired enantiomer is separated from soluble substances, wherein the desired enantiomer is present as an insoluble material; and
iii) Isolating the desired enantiomer obtained in step (ii) by filtration.
|