| CPC C07D 487/04 (2013.01) [A61K 31/428 (2013.01); A61K 31/433 (2013.01); A61K 31/522 (2013.01); C07D 249/12 (2013.01); C07D 277/82 (2013.01); C07D 285/08 (2013.01); C07D 405/12 (2013.01)] | 13 Claims |
|
1. A pharmaceutical composition comprising
an amount of a compound of Formula A
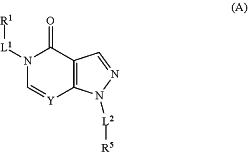 or a tautomer thereof and/or a pharmaceutically acceptable salt thereof effective to inhibit fascin expression or fascin activity, and
a pharmaceutically acceptable excipient;
wherein in Formula A:
Y is N or CR;
R is hydrogen, halo, or lower alkyl;
R1 is phenyl, 5-membered heteroaryl or 6-membered heteroaryl, wherein the phenyl, 5-membered heteroaryl or 6-membered heteroaryl is optionally substituted with 1 to 3 R6;
L1 is selected from the group consisting —(C(R8)2)j—,
—(C(R8)2)q—C(O)—(C(R8)2)r—,
—(C(R8)2)q—C(O)N(R8)—(C(R8)2)r—,
—(C(R8)2)q—N(R8)C(O)—(C(R8)2)r—,
—(C(R8)2)q—N(R8)S(O)2—(C(R8)2)r—, —(CH2)q—S(O)2N(R8)—(CH2)r—, —S—,
—O—, and —NR8—;
j is 1, 2, or 3;
q is 0 or 1;
r is 0 or 1;
L2 is selected from the group consisting a covalent bond,
—C(O)N(R8)—, —N(R8)C(O)—, —N(R8)S(O)2—, and —S(O)2N(R8)—;
R5 is phenyl, 5-membered heteroaryl, 6-membered heteroaryl, 5-membered heterocycloalkyl or 6-membered heterocycloalkyl; wherein the phenyl is substituted with 1 to 4 R2, and the 5-membered heteroaryl or 6-membered heteroaryl is optionally substituted with 1 to 4 R2, wherein each R2 is independently selected from the group consisting of lower alkyl, lower haloalkyl, —OH, —OR7, —SH, —SR7, —NR10R10, halo, cyano, nitro, —COH, —COR7, —CO2H, —CO2R7, —CoNR10R10, OCOR7, —OCO2R7, —OCONR10R10, —NR10COR10, —NR10CO2R10, —SOR7, —SO2R7, —SO2NR10R10, and —NR1SO2R7;
each R6 is independently selected from the group consisting of halo and lower alkyl optionally substituted with 1-3 halo; or two adjacent R6 on a phenyl ring form a 5- or 6-membered cycloalkyl or heterocycloalkyl fused with the phenyl ring;
R7 is lower alkyl;
R8 is hydrogen; and
each R10 is independently hydrogen or lower alkyl, or two R10 together with the atom(s) attached thereto form a 4- to 6-membered ring, with the proviso that when R5 is phenyl and L2 is a covalent bond, then R5 is substituted with 1 to 4 R2.
|