| CPC C07D 471/14 (2013.01) [A61K 31/4353 (2013.01); A61P 35/00 (2018.01); C07D 471/04 (2013.01); C07D 471/22 (2013.01); C07D 487/14 (2013.01)] | 9 Claims |
|
1. A method for treating cancer in a subject in need thereof, wherein the cancer is leukemia or breast cancer, comprising administering to the subject a compound of having the structure of Formula III(A)(1), III(B)(1), or III(C)(1), or a pharmaceutically acceptable salt, hydrate, or tautomer thereof:
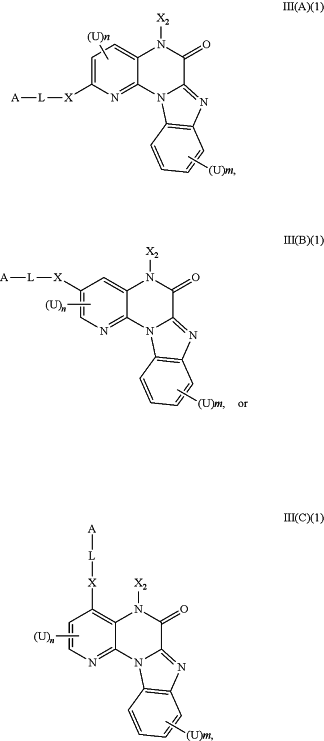 wherein:
L is a bond, C1-C10 alkylene, C1-C10 heteroalkylene, C2-C10 alkenylene, or C2-C10 heteroalkenylene linker, each of which may be optionally substituted with one or more substituents selected from the group consisting of halogen, oxo (═O), and C1-C6 alkyl;
A is heterocycloalkyl, heteroaryl, or NR4R5,
R4 and R5 are independently H, optionally substituted C1-C8 alkyl, C2-C8 heteroalkyl, C2-C8 alkenyl, C2-C8 heteroalkenyl, C2-C8 alkynyl, C2-C8 heteroalkynyl, C1-C8 acyl, C2-C8 heteroacyl, C6-C10 aryl, C5-C12 heteroaryl, C7-C12 arylalkyl, or C6-C12 heteroarylalkyl group; or R4 and R5 can be linked to form a 3-8 membered ring, optionally containing one or more N, O or S; and each R4 and R5 groups, and each ring formed by linking R4 and R5 groups together, is optionally substituted with one or more substituents selected from halo, ═O, ═N—CN, ═N—OR′, ═NR′, OR′, N(R′)2, SR′, SO2R′, SO2NR′2, NR′SO2R′, NR′CONR′2, NR′ COOR′, NR′COR′, CN, COOR′, CON(R′)2, OOCR′, COR′, and NO2,
wherein each R′ is independently H, C1-C6 alkyl, C2-C6 heteroalkyl, C1-C6 acyl, C2-C6 heteroacyl, C6-C10 aryl, C5-C10 heteroaryl, C7-C12 arylalkyl, or C6-C12 heteroarylalkyl, each of which is optionally substituted with one or more groups selected from halo, C1-C4 alkyl, C1-C4 heteroalkyl, C1-C6 acyl, C1-C6 heteroacyl, hydroxy, amino, and ═O; wherein two R′ can be linked to form a 3-7 membered ring optionally containing up to three heteroatoms selected from N, O and S;
X is NR6, O, or S;
R6 is H, optionally substituted C1-C8 alkyl, C2-C8 heteroalkyl, C2-C8 alkenyl, C2-C8 heteroalkenyl, C2-C8 alkynyl, C2-C8 heteroalkynyl, C1-C8 acyl, C2-C8 heteroacyl, C6-C10 aryl, C5-C12 heteroaryl, C7-C12 arylalkyl, or C6-C12 heteroarylalkyl group; or
R6 can be linked to R4 or R5 to form a 3-8 membered ring;
or A-L-X— is:
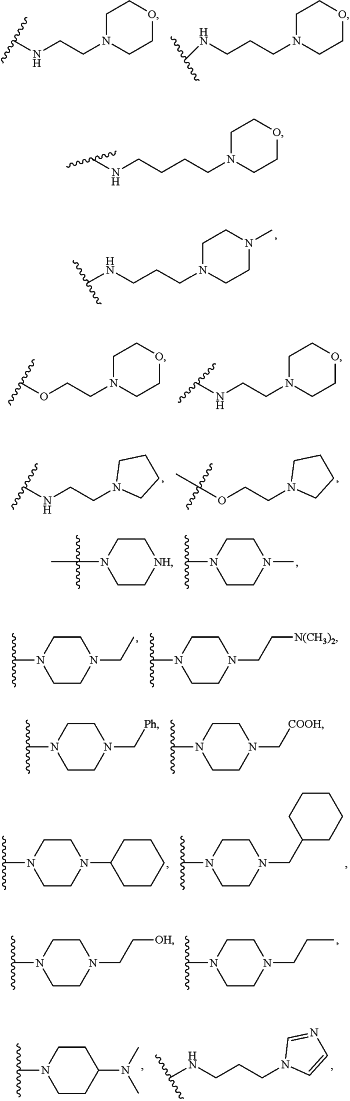 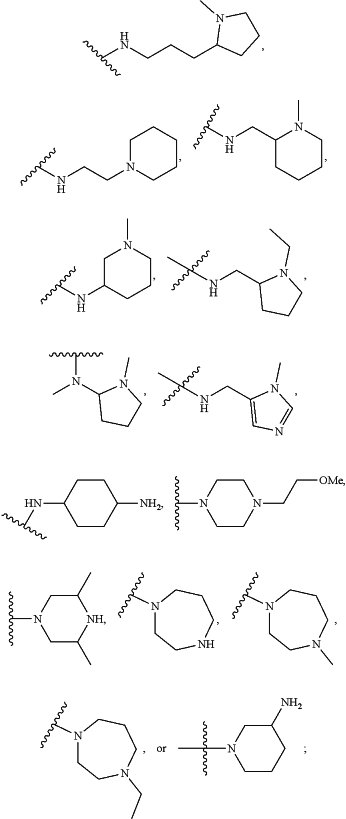 X2 is hydrogen, or an optionally substituted C1-C8 alkyl, C2-C8 heteroalkyl, C2-C8 alkenyl, C2-C8 heteroalkenyl, C2-C8 alkynyl, C2-C8 heteroalkynyl, C1-C8 acyl, C2-C8 heteroacyl, C6-C10 aryl, C5-C12 heteroaryl, C7-C12 arylalkyl, or C6-C12 heteroarylalkyl; or
X2 is H, C1-C10 alkyl, C1-C10 heteroalkyl, C2-C10 alkenyl, or C2-C10 heteroalkenyl, each of which is optionally substituted with one or more halogens, ═O, or an optionally substituted 3-7 membered carbocyclic or heterocyclic ring; and
(U)n and (U)m are each independently H, halogen, CF3, CN, OR7, NR8R9, SR7, SO2NR8R9, C1-C10 alkyl, C1-C10 heteroalkyl, C2-C10 alkenyl, or C2-C10 heteroalkenyl, each of which may be optionally substituted with one or more halogens, ═O, or an optionally substituted 3-7 membered carbocyclic or heterocyclic ring;
wherein each R7, R8 and R9 is independently selected from H, C1-C6 alkyl, C2-C6 heteroalkyl, C1-C6 acyl, C2-C6 heteroacyl, C6-C10 aryl, C5-C10 heteroaryl, C7-C12 arylalkyl, or C6-C12 heteroarylalkyl; and
wherein the term “optionally substituted” means that the referenced group, if no optional substituents are specifically described for the referenced group, is substituted with a substituent selected from halogen, —CN, —NH2, —OH, —NH(CH3), —N(CH3)2, —CH3, —CH2CH3, —CF3, —OCH3, and —OCF3.
|