| CPC C07D 471/04 (2013.01) [A61K 31/4375 (2013.01); A61P 35/00 (2018.01)] | 11 Claims |
|
1. A compound of Formula (I):
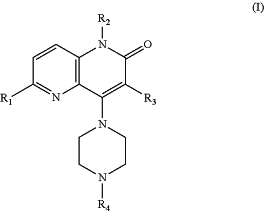 or a salt thereof, wherein:
R1 is H, Cl, Br, —CN, C1-4 alkyl, C2-3 alkenyl, C1-3 alkoxy, —C(O)OH, —C(O)O(C1-3 alkyl), —C(O)NRaRa, —NRaRa, —NRaC(O)O(C1-4 alkyl), or —NRaC(O)NRa(C1-4 alkyl);
each Ra is independently H or C1-2 alkyl;
R2 is C1-6 alkyl, C1-4 cyanoalkyl, C1-4 fluoroalkyl, C2-4 alkenyl, —(CH2)1-3CH═CF2, C3-5 alkynyl, —(CH2)1-4O(C1-3 alkyl), —(CH2)1-4O(CH2)1-3O(C1-3 alkyl), —(CH2)1-3C(O)(C1-3 alkyl), —(CH2)1-3C(O)O(C1-3 alkyl), —(CH2)1-3Rb, —(CH2)1-3ORb, or —(CH2)1-3OCH2Rb;
Rb is C3-6 cycloalkyl or dioxanyl, each substituted with zero to 2 substituents independently selected from F, —CN, —CH3, and —OCH3;
R3 is H, F, Cl, Br, —CN, C1-3 alkyl, C1-3 fluoroalkyl, —NO2, —C(O)(C1-3 alkyl), —C(O)O(C1-3 alkyl), or —C(O)(C1-3 fluoroalkyl);
R4 is:
(a) 2,3-dihydro-1H-indenyl substituted with zero to 2 substituents independently selected from F, Cl, —OH, C1-2 alkyl, C1-2 fluoroalkyl, C1-2 alkoxy, and —OCH2CH═CH2; or
(b) —CH2Ry, —C(CH3)2Ry, —CHRxRy, —CH2CH(OH)Rx, —CH(CH3)(CH2CH2OCH3), or C3-6 cycloalkyl substituted with fluorophenyl;
Rx is C1-6 alkyl, C1-3 hydroxyalkyl, C1-2 aminoalkyl, C3-6 cycloalkyl, or phenyl substituted with zero to 2 substituents independently selected from F, Cl, —OH, C1-3 alkyl, C1-2 fluoroalkyl, C1-2 alkoxy, —OCH2CH═CH2, and —OCH2C≡CH;
Ry is 1,3-benzodiazolyl, indazolyl, indolyl, indolinyl, naphthalenyl, oxoindolinyl, pyridinyl, pyrimidinyl, or phenyl, each substituted with zero to 3 substituents independently selected from F, Cl, Br, —OH, —CN, C1-6 alkyl, C1-3 fluoroalkyl, C1-3 alkoxy, C1-3 fluoroalkoxy, —OCH2CH═CH2, —OCH2C≡CH, —OCH2(cyanopyridinyl), —NRcRc, —NRaS(O)2(C1-3 alkyl), —NRaC(O)(C1-3 alkyl), —NRaC(O)O(C1-4 alkyl), —NRaC(O)Rd, —NRaC(O)NRaRd, and Rd;
each Rc is independently H or C1-2 alkyl; and
Rd is phenyl substituted with zero to 1 substituent selected from Cl, —CH3, and —OCH3.
|