| CPC C07D 471/04 (2013.01) [A61K 31/496 (2013.01); A61K 31/497 (2013.01); A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61K 31/5025 (2013.01); A61K 31/5377 (2013.01); C07D 401/06 (2013.01); C07D 403/06 (2013.01); C07D 403/14 (2013.01); C07D 413/14 (2013.01); C07D 471/10 (2013.01); C07D 487/04 (2013.01); C07D 487/10 (2013.01); C07D 519/00 (2013.01)] | 21 Claims |
|
1. A protected form of formula (I):
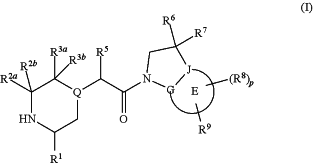 or a tautomeric or stereochemically isomeric form, N-oxide, pharmaceutically acceptable salt, or solvate thereof, wherein
Ring E represents a 6 membered aromatic carbocyclic or heterocyclic group;
R1 is selected from C1-4 alkyl, C2-4 alkenyl and —(CH2)s-C3-8 cycloalkyl, wherein said C1-4 alkyl, C2-4 alkenyl, and C3-8 cycloalkyl may be optionally substituted by one or more Ra groups;
Ra is selected from halogen, —OH and —O—C1-6alkyl;
R3a and R3b are independently selected from hydrogen, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, —C(═O)NH(2-q)(C1-6 alkyl)q, —(CH2)s-(3-12 membered heterocyclyl), —(CH2)s—C3-12 carbocyclyl, —C(═O)-(3-12 membered heterocyclyl), and —C(═O)—C3-12 carbocyclyl,
or R3a and R3b groups, together with the carbon atom to which they are attached, can join to form a 3-10 membered saturated carbocyclyl or heterocyclyl group, wherein said C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, heterocyclyl and carbocyclyl groups may be optionally substituted by one or more Rb groups;
R5 is selected from hydrogen or C1-6 alkyl;
R6 and R7 are independently selected from hydrogen, C1-8 alkyl, C2-8 alkenyl, C2-8 alkynyl, —Y—C3-12 carbocyclyl, —Z-(3-12 membered heterocyclyl), —(CRxRy)s—O—Rz, —O—(CRxRy)n—ORz —(CH2)s—CN, —S(O)q-R, —C(═O)Rx, —C(═S)Rx, —C(═N)Rx, —(CRxRy)s—C(═O)ORz, —(CRxRy)s—O—C(═O)—Rz, —(CRxRy)s—C(═O)NRxRy, —(CH2)s—NRxC(═O)Ry, —(CH2)s—OC(═O)NRxRy, —(CH2)s—NRxC(═O)ORy, —(CH2)s—NRxRy, —NRx—(CH2)s—Rz, —(CRxRy)s—C(═S)NRz, —(CRxRy)s—C(═N)NRz, —(CH2)s—O—C(═O)—C1-4alkyl-NRxRy, —(CH2)s—NRx—(CH2)n—O—C(═O)—Rz, —(CH2)s—NRx—(CH2)s—SO2—Ry, —(CH2)s—NH—SO2—NRxRy and —(CH2)s-SO2NRxRy groups,
or R6 and R7 groups, together with the carbon atom to which they are attached, can join to form a 3-10 membered fully or partially saturated carbocyclyl or heterocyclyl group, which may be optionally fused to a 5-6 membered aromatic carbocyclyl or heterocyclyl ring,
wherein said C1-8 alkyl, C2-8 alkenyl and C2-8 alkynyl groups may be optionally substituted by one or more Rb groups and wherein said carbocyclyl and heterocyclyl groups may be optionally substituted by one or more Rb groups;
R8 and R9 are independently selected from hydrogen, halogen, C1-8 alkyl, C2-8 alkenyl, C2-8 alkynyl, —Y—C3-12 carbocyclyl, —Z-(3-12 membered heterocyclyl), —(CRxRy)s—O—Rz, —O—(CRR)n-ORz, =0, ═S, nitro, Si(Rx)4, —(CH2)s—CN, —S(O)q—(CRxRy)s—Rz, —C(═O)Rx, —C(═S)Rx, —C(═N)Rx, —(CRxR)s—C(═O)ORz, —(CRxR)s—O—C(═O)—Rz, —(CRxRy)s—C(═O)NRxRy, —(CH2)s—NRxC(═O)Ry, —(CH2)s—OC(═O)NRxRy, —(CH2)s—NRxC(═O)ORy, —(CH2)s—NRxRy, —NRx—(CH2)s—Rz, —(CRxRy)s—C(═S)NRz, —(CRxRy)s—C(═N)NRx, —S(O)(=NRx)Ry, —(CH2)s—O—C(═O)—C1-4alkyl-NRxRy, —(CH2)s—NRx—(CH2)n—O—C(═O)—Rz, —(CH2)s—NRx—(CH2)s—SO2—Ry, —(CH2)s—NH—SO2—NRxRy, —(CH2)s—SO2NRxRy groups and —P(═O)(Rx)2, wherein said C1-8 alkyl, C2-8 alkenyl and C2-8 alkynyl groups may be optionally substituted by one or more Rb groups and wherein said carbocyclyl and heterocyclyl groups may be optionally substituted by one or more Rb groups;
Rb is independently selected from halogen, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, —(CH2)s—C3-8 cycloalkyl, —(CH2)s—C3-8 cycloalkenyl, —(CH2)s-phenyl, —(CH2)s-(4-7 membered saturated heterocyclyl), —(CRxRy)s—O—Rz, —O—(CRxRy)n—ORz, haloC1-6 alkyl, haloC1-6 alkoxy, C1-6 alkanol, ═O, ═S, nitro, Si(Rx)4, —(CH2)s—CN, —S(O)q—Rx, —C(═O)Rx, —(CRxR)s—C(═O)ORz, —(CRxRy)s—O—C(═O)—Rz, —(CRxRy)s—C(═O)NRxRy, —(CH2)s—NRxC(═O)Ry, —(CH2)s—OC(═O)NRxRy, —(CH2)s—NRxC(═O)ORy —(CH2)s—NRxRy, —NRx—(CH2)s—Rz, —(CH2)s—O—C(═O)—C1-4alkyl-NRxRy, —(CH2)s—NRx—(CH2)n—O—C(═O)—Rz, —(CH2)s—NRx—(CH2)s—SO2—Ry, —(CH2)s—NH—SO2—NRxRy, —(CH2)s—SO2NRxRy groups and —P(═O)(Rx)2, wherein said C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-8 cycloalkyl, C3-8 cycloalkenyl and heterocyclyl groups may be optionally substituted by one or more Rx groups;
Rx, Ry and Rz independently represent halogen, hydrogen, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, —(CH2)s—C3-8 cycloalkyl, —(CH2)s—C3-8 cycloalkenyl, —(CH2)s-phenyl, —(CH2)s-(4-7 membered saturated heterocyclyl), C1-6 alkanol optionally substituted with one or more halo, —C(═O)OC1-6 alkyl, hydroxy, C1-6 alkoxy, haloC1-6 alkyl, —(CH2)n—O—C1-6alkyl, —C(═O)—(CH2)n—C1-6 alkoxy, —C(═O)—C1-6alkyl, —(CH2)s—CN, C1-6 alkyl-N(H)2-q(C1-6alkyl)q, —N(H)2-q(C1-6alkyl)q, —C(═O)—N(H)2-q (C1-6alkyl)q, —(CH2)s—NH—SO2—N(H)2-q (C1-6alkyl)q, —(CH2)s—N(C1-4alkyl)-SO2—N(H)2-q(C1-6alkyl)qand —(CH2)s—O—C(═O)—C1-4alkyl-N(H)2-q(C1-6alkyl)q, and when attached to nitrogen, carbon, silicon or phosphorus atom Rx and Ry may join to form a 3-7 membered ring optionally containing one or two additional heteroatoms selected from O, N, S and oxidised forms of N or S;
Y and Z are independently selected from a bond, —(CRxRy)m—, —C(═CRx)—, —C(═O)—, —NRx—, —C(═O)NRx—, —NRxC(═O)—, —(CRxR)q—O—, —O—(CRxRy)q—, —S(O)2—NH—, —NH—S(0)2- and —S(O)q—;
s independently represents an integer from 0-4;
n independently represents an integer from 1-4;
p independently represents an integer from 0-4;
q represents an integer from 0-2; and
m represents an integer from 1-2.
|