| CPC C07D 403/14 (2013.01) [C07D 239/47 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 405/14 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 491/107 (2013.01); C07D 498/08 (2013.01)] | 26 Claims |
|
1. A method of treating a LRRK2-mediated disease or disorder selected from the group consisting of Parkinson's disease, Lewy body dementia, Alzheimer's disease, L-DOPA induced dyskinesia, kidney cancer, breast cancer, prostate cancer, a blood cancer, papillary renal and thyroid carcinomas, lung cancer, acute myelogenous leukemia, leprosy, Crohn's disease, amyotrophic lateral sclerosis, rheumatoid arthritis, and ankylosing spondylitis, comprising administering a therapeutically effective amount of a compound having the following Structure (I):
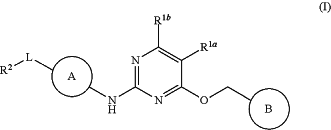 or a pharmaceutically acceptable salt, or stereoisomer thereof, wherein:
A is phenylene or 5 or 6-membered heteroarylene, each of which is optionally substituted with one or more substituents selected from halo, C1-C6 alkyl, C3-C8 cycloalkyl, and C1-C6 alkoxy;
B is C3-C8 monocyclic cycloalkyl, C6-C10 spirocyclic cycloalkyl, C6-C10 fused-multicyclic cycloalkyl, C6-C10 bridged-multicyclic cycloalkyl, 3-8-membered monocyclic heterocyclyl, 3-8-membered monocyclic heterocyclylalkyl, 6-10-membered spirocyclic heterocyclyl, 6-10-membered fused-multicyclic heterocyclyl or 6-10-membered bridged-multicyclic heterocyclyl, each of which is optionally substituted with one or more substituents selected from amino, halo, hydroxyl, oxo, cyano, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxylalkyl, C1-C6 aminoalkyl, C1-C6 alkylaminylalkyl, C1-C6 alkoxy; C1-C6 haloalkoxy, C1-C6 alkylcarbonyl, C1-C6 haloalkylcarbonyl, C1-C6 alkylaminyl, C1-C6 haloalkylaminyl, C1-C6 alkylcarbonylaminyl, C1-C6 haloalkylcarbonylaminyl, C3-C8 cycloalkyl, C3-C8 cycloalkylcarbonyl, C3-C8 halocycloalkylaminyl, and C3-C8 cycloalkylcarbonylaminyl;
L is a direct bond, CH2 or C═O;
R1a and R1b are each independently H, halo, cyano, unsubstituted C1-C6 alkyl, unsubstituted C1-C6 haloalkyl, unsubstituted C1-C6 alkoxy or unsubstituted C3-C8 cycloalkyl; and
R2 is C1-C6 alkyl, C3-C8 cycloalkyl, 3-8-membered heterocyclyl, 6-10-membered spirocyclic heterocyclyl, 6-10-membered fused-multicyclic heterocyclyl or 6-10-membered bridged-multicyclic heterocyclyl each of which is optionally substituted with one or more substituents selected from halo, cyano, C1-C6 alkyl, hydroxyl, alkoxy, and C1-C6 haloalkyl, to a mammal in need thereof.
|