| CPC C07D 403/04 (2013.01) [C07D 207/50 (2013.01); C07D 401/04 (2013.01); C07D 401/14 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 471/04 (2013.01)] | 1 Claim |
|
1. A compound of Formula I:
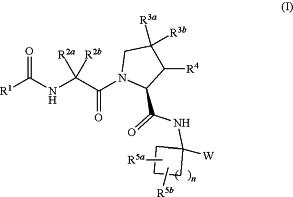 or a pharmaceutically acceptable salt, solvate, solvate of the salt or prodrug thereof wherein:
W is selected from the group consisting of: —B(OH)2 and —C(O)C(O)NR7R8;
R1 is selected from the group consisting of:
(a) —(CH2)0-6-aryl, and
(b) —(CH2)0-6-heteroaryl,
wherein the aryl and heteroaryl of choices (a) and (b) are each optionally substituted with 1 to 3 substituents independently selected from the group consisting of:
(i) -halogen,
(ii) —CN,
(iii) —C1-6alkyl,
(iv) —C0-6alkyl-R6,
(v) —C2-6alkenyl,
(vi) —C2-6alkynyl,
(vii) —C(O)R7,
(viii) —CO2R7,
(ix) —CONR7R8,
(x) —OH,
(xi) —O—C1-6alkyl,
(xii) —O—C0-6alkyl-R6,
(xiii) —SH,
(xiv) —S(O)p—C1-6alkyl,
(xv) —S(O)p—C0-6alkyl-R6,
(xvi) —S(O)2NR7R8,
(xvii) —NO2,
(xviii) —NR7R8,
(xix) —NHC(O)R7,
(xx) —NHC(O)OR7,
(xxi) —NHC(O)NR7R8,
(xxii) —NHSO2C1-6alkyl, and
(xxiii) —NHSO2C0-6alkyl-R6,
(xxiv) —CONH(CH2)2-4—[O(CH2)2-4]mOC1-4alkyl,
wherein each of the alkyl group of choices (iii), (iv), (xi), (xii), (xiv), (xv), (xxii), (xxiii) and (xxiv) is optionally substituted with 1 to 5 substituents independently selected from -halogen, -haloC1-4alkyl, —COR7, —CO2R7, —CONR7R8, —NR7R8, —OH, —O—C1-4alkyl, —SH and —S—C1-4alkyl;
R2a and R2b are independently selected from the group consisting of:
(a) —H,
(b) —C1-8alkyl, and
(c) —C0-6alkyl-R6,
wherein each of the alkyl group of choices (b) and (c) is optionally substituted with 1 to 5 substituents independently selected from:
(i) -halogen,
(ii) -haloC1-4alkyl,
(iii) —NR7R8,
(iv) —OH,
(v) —O—C1-4alkyl,
(vi) —SH,
(vii) —S—C1-4alkyl,
(viii) —NR7SO2C1-4alkyl,
(ix) —NR7C(O)R7, and
(x) —NR7C(O)OR7,
with the proviso that R2a and R2b are not both H;
R3a is H, and R3b is selected from the group consisting of:
(a) —H,
(b) —OH,
(c) -heteroaryl,
(d) —O-heteroaryl,
(e) -heterocycle,
(f) -aryl, and
(g) —O-aryl;
wherein each of the heteroaryl of choices (c) and (d), the heterocycle of choice (e) and the aryl of choices (f) and (g) is optionally substituted with 1 to 3 groups independently selected from the group consisting of:
(i) -halogen,
(ii) —OH,
(iii) —CR10R11R12,
(iv) —(CH2)0-3—NHSO2—C1-4alkyl,
(v) —(CH2)0-3—NHSO2-C3-12cycloalkyl,
(vi) —(CH2)0-3—SO2—C1-4alkyl,
(vii) —(CH2)0-3—C(O)O-R7, and
(viii) —CN; and
wherein the heterocycle of choice (e) is additionally optionally substituted with 1 to 2 oxo groups; or
R3a and R3b together represent oxo;
R4 is selected from a group consisting of
(a) —H,
(b) —C1-4alkyl,
(c) -haloC1-4alkyl,
(d) —O—C1-4alkyl, and
(e) —O-haloC1-4alkyl;
R5a and R5b are independently selected from a group consisting of
(a) —H,
(b) —C1-4alkyl,
(c) -halogen,
(d) —OH,
(e) —O—C1-4alkyl,
(f) —SH, and
(g) —S—C1-4alkyl, or
R5a, R5b and the atom(s) to which they are attached together form a 3- to 6-membered cycloalkyl or a 4- to 6-membered heterocycle having a heteroatom selected from O and S(O)p, and wherein said cycloalkyl or heterocycle is optionally substituted with 1 to 2 groups independently selected from halogen, —C1-4alkyl, —OH, —O—C1-4alkyl, —SH, -S-C1-4alkyl;
R6 is selected from the group consisting of:
(a) —C3-12cycloalkyl,
(b) —aryl,
(c) -heteroaryl, and
(d) -heterocyclyl,
wherein each of choices (a) to (d) is optionally substituted with 1 to 3 substituents independently selected from the group consisting of:
(i) —C1-4alkyl,
(ii) -halogen,
(iii) —NR7R8,
(iv) —OH,
(v) —O—C1-4alkyl,
(vi) —SH, and
(vii) —S—C1-4alkyl;
wherein each of the alkyl group of choices (i), (v) and (vii) is optionally substituted with 1 to 5 substituents independently selected from -halogen, -haloC1-4alkyl, —OH, —O—C1-4alkyl, —SH and —S—C1-4alkyl;
each R7 and each R8 are independently selected from the group consisting of:
(a) —H,
(b) —C1-6alkyl,
(c) —C0-6alkyl-C3-12cycloalkyl,
(d) —C0-6alkyl-heterocyclyl,
(e) —C0-6alkyl-heteroaryl,
(f) —C0-6alkyl-aryl,
(g) —C2-6alkenyl, and
(h) —C2-6alkynyl,
wherein the alkyl group of choices (b)-(f), the alkenyl group of choice (g) and the alkynyl group of (h) are each optionally substituted with 1 to 3 groups independently selected from:
(i) -halogen,
(ii) —C(O)C1-4alkyl,
(iii) —C(O)NH2,
(iv) —C(O)NH(C1-4alkyl),
(v) —C(O)N(C1-4alkyl)(C1-4alkyl)
(vi) —OH,
(vii) —OC1-4alkyl,
(viii) —SH,
(ix) —S(O)pC1-4alkyl,
(x) —NH2,
(xi) —NH(C1-4alkyl), and
(xii) —N(C1-4alkyl)(C1-4alkyl); or
R7, R8 and the nitrogen atom to which they are attached together form a 3- to 7-membered monocyclic or 6- to 11-membered bicyclic heterocycle optionally having an additional heteroatom selected from O, S(O)p, and NR9, and wherein said heterocycle is optionally substituted with 1 to 2 halogen;
R9 is selected from the group consisting of:
(a) —H,
(b) —C1-4alkyl,
(c) —C(O)—C1-4alkyl,
(d) —C(O)NH2,
(e) —C(O)—NH(C1-4alkyl),
(f) —C(O)—N(C1-4alkyl)2,
(g) —C(O)O—C1-4alkyl; and
(h) —C(O)O—C1-4alkyl-aryl;
R10, R11, and R12 are independently selected from the group consisting of: H, halogen, —OH and —C1-6 alkyl; or
R10, R11 and the atom to which they are attached together form a C3-12cycloalkyl or a heterocyclyl group;
n is 0, 1, 2, 3, 4 or 5;
m is 1-25; and
p is 0, 1 or 2.
|