| CPC C07D 401/12 (2013.01) [A61K 31/404 (2013.01); A61K 31/429 (2013.01); A61K 31/437 (2013.01); A61K 31/438 (2013.01); A61K 31/444 (2013.01); A61K 31/4709 (2013.01); A61K 31/4745 (2013.01); A61K 31/496 (2013.01); A61K 31/498 (2013.01); A61K 31/4985 (2013.01); A61K 31/519 (2013.01); A61K 45/06 (2013.01); A61P 31/04 (2018.01); A61P 31/06 (2018.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 487/10 (2013.01); C07D 513/04 (2013.01); C07D 519/00 (2013.01)] | 18 Claims |
|
1. A compound of formula (IA) for use in the treatment of tuberculosis
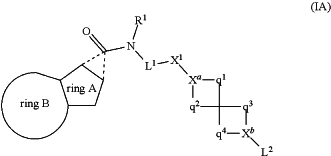 wherein
R1 represents C1-6 alkyl or hydrogen;
L1 represents a linker group —C(Ra)(Rb)—;
X1 represents an aromatic linker group that is phenylene (which linker group may itself be optionally substituted by one or more substituents selected from fluoro, —OH, —OC1-6 alkyl and C1-6 alkyl, wherein the latter two alkyl moieties are themselves optionally substituted by one or more fluoro atoms);
Ra and Rb independently represent hydrogen or C1-6 alkyl (optionally substituted by one or more fluoro atoms);
Xa represents CH or N;
Xb represents CH, N, O (in which case L2 is not present) or C═O (in which case L2 is also not present);
q1 represents —CH2—, —CH2—CH2—, —O—CH2—, or “—”;
q2 represents —CH2—, or —CH2—CH2—;
q3 represents —CH2—, or —CH2—CH2—;
q4 represents —CH2—, or —CH2—CH2—;
when Xb represents O or C═O, then L2 is not present;
when Xb represents C(Ra) or N, then L2 may represent hydrogen, halo, —ORf, —C(O)—Rg, C1-6 alkyl (optionally substituted by one or more halo), or an aromatic group (optionally substituted by one or more substituents selected from halo, C1-6 alkyl (itself optionally substituted by one or more substituents selected from fluoro, —CF3 and/or —SF5), —OC1-6alkyl (itself optionally substituted by one or more fluoro atoms), —O-phenyl (itself optionally substituted by halo, C1-6alkyl, C1-6fluoroalkyl and/or —OC1-6alkyl) or —SF5); or, when Xb is N, L2 represents —S(O)2—C1-6alkyl optionally substituted by one or more fluoro atoms;
Rf represents hydrogen, C1-6 alkyl (optionally substituted by one or more fluoro) or an aromatic group (itself optionally substituted by one or more substituents selected from halo, C1-6alkyl and —OC1-6alkyl, where the latter two alkyl moieties may themselves be optionally substituted by one or more fluoro atoms);
Rg represents hydrogen or C1-6alkyl (optionally substituted by one or more substituents selected from fluoro, or —OC1-3 alkyl, which latter moiety is also optionally substituted by one or more fluoro atoms) or an aromatic group (optionally substituted by one or more substituents selected from halo, C1-6 alkyl or —OC1-6alkyl);
wherein the combined ring systems, i.e. Ring A and Ring B may be represented as follows:
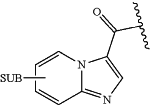 either ring A and/or ring B may be optionally substituted by one or more substituents selected from: halo, C1-6 alkyl (optionally substituted by one or more halo) and/or —OC1-6alkyl (itself optionally substituted by one or more fluoro atoms),
or a pharmaceutically-acceptable salt thereof.
|