| CPC C07D 401/10 (2013.01) [C07D 209/12 (2013.01); C07D 261/20 (2013.01); C07D 307/80 (2013.01); C07D 333/64 (2013.01); C07D 401/12 (2013.01); C07D 405/10 (2013.01)] | 17 Claims |
|
1. A method of treating a disease or condition characterized by aberrant complement system activity, comprising administering to a subject in need thereof a therapeutically effective amount of a compound, or a pharmaceutically acceptable salt thereof, selected from the group consisting of:
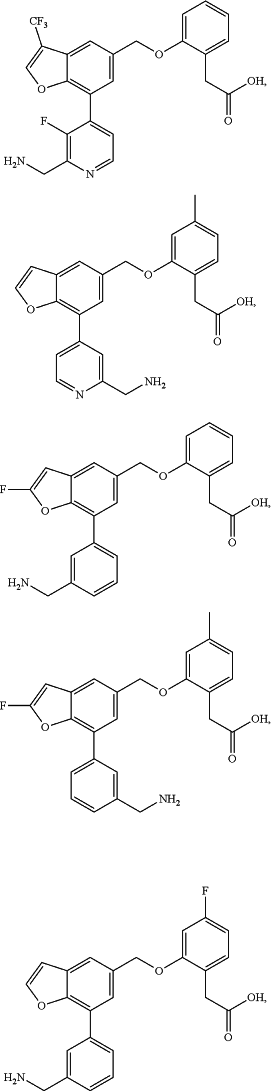 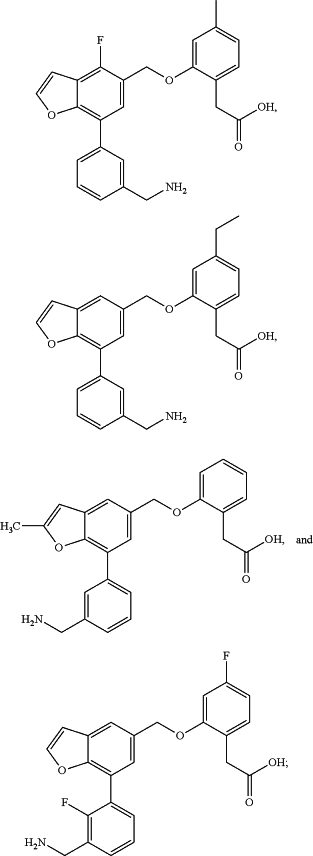 wherein the disease or condition characterized by aberrant complement system activity is selected from the group consisting of age-related macular degeneration (AMD), atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, dense deposit disease, paroxysmal nocturnal hemoglobinuria (PNH), organ transplant rejection, myasthenia gravis, neuromyelitis optica, cold agglutinin disease, catastrophic antiphospholipid syndrome, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), IgA nephropathy, warm autoimmune hemolytic anemia, and focal segmental glomerulosclerosis.
|