| CPC C07C 405/0016 (2013.01) [B01J 23/462 (2013.01); C07C 2601/10 (2017.05)] | 10 Claims |
|
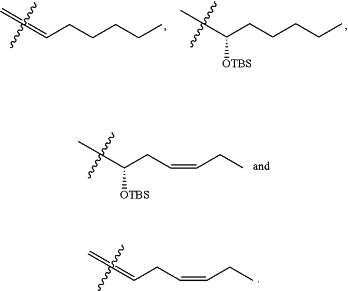
1. A method for producing at least one Δ12-Prostaglandin J product represented by Formula (IV),
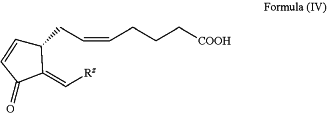 wherein: Rz is selected from
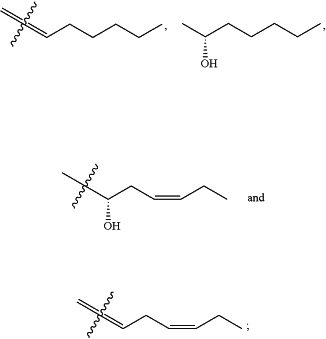 and wherein at least one carbon-carbon double bond has a Z/E-selectivity of 95/5, or 96/4, or 97/3, or 98/2, or 99/1, or >99/<1;
comprising, submitting an alcohol product of Formula (III)
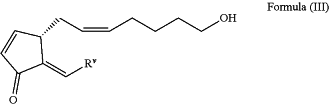 to oxidation conditions; wherein the alcohol product of Formula (III) is formed during a cross-metathesis reaction between a substrate represented by Formula (II)
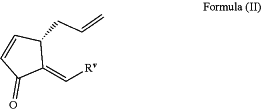 and cis-5-octen-1-ol in the presence of a ruthenium olefin metathesis catalyst; and wherein Rv is selected from
 |