| CPC B01J 23/80 (2013.01) [B01J 23/005 (2013.01); B01J 37/009 (2013.01); B01J 37/0236 (2013.01); B01J 37/031 (2013.01); B01J 37/06 (2013.01); B01J 37/08 (2013.01); C07C 5/48 (2013.01); C07C 2523/80 (2013.01)] | 8 Claims |
|
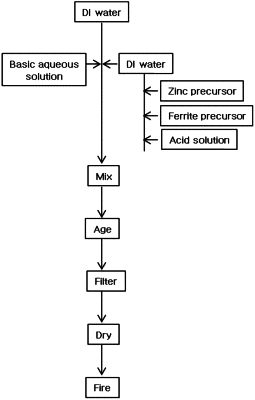
1. A method for preparing a zinc ferrite-based catalyst, the method comprising:
obtaining a precipitate by bringing a metal precursor solution comprising a zinc precursor, a ferrite precursor, a solution containing an acid in an amount from 1 wt % to 30 wt % based on a combined weight of the zinc precursor and the ferrite precursor, and water into contact with a basic aqueous solution;
filtering the precipitate;
drying the filtered precipitate; and
firing the dried precipitate,
wherein the solution containing the acid comprises one or more of nitric acid and hydrocarbon acid,
wherein the zinc ferrite-based catalyst comprises Cl− ions, and a content of the Cl− ions is 1,100 ppm or less based on a total weight of the zinc ferrite-based catalyst.
|