| CPC A61N 1/36096 (2013.01) [A61B 18/02 (2013.01); A61N 1/0551 (2013.01); A61B 18/04 (2013.01); A61B 18/08 (2013.01); A61B 18/18 (2013.01); A61B 18/22 (2013.01); A61B 2018/0022 (2013.01); A61B 2018/00434 (2013.01); A61B 2018/00511 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00666 (2013.01); A61B 2018/00791 (2013.01); A61B 2018/00875 (2013.01); A61B 2018/0212 (2013.01); A61N 2/006 (2013.01); A61N 7/00 (2013.01)] | 20 Claims |
|
1. A method comprising:
prior to a patient experiencing a potential traumatic event, assessing the patient for risk of developing post-traumatic stress disorder (PTSD) in response to experiencing the potential traumatic event;
identifying the patient as being at risk for developing PTSD from experiencing the potential traumatic event; and
in response to identifying the patient as being at risk for developing PTSD from experiencing the potential traumatic event, executing a renal denervation procedure to reduce the risk associated with developing PTSD, the renal denervation procedure comprising:
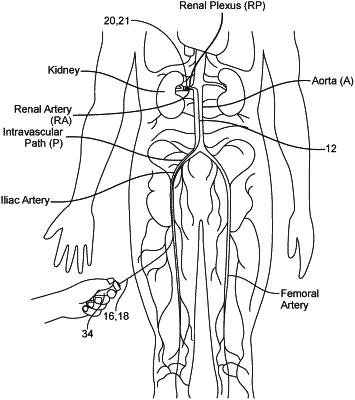
intravascularly positioning a catheter carrying a neuromodulation assembly adjacent to a renal sympathetic nerve in the patient;
delivering energy to the renal sympathetic nerve via the neuromodulation assembly to at least partially ablate the renal sympathetic nerve to attenuate neural traffic along the renal sympathetic nerve; and
removing the catheter and the neuromodulation assembly from the patient after treatment.
|