| CPC A61K 51/044 (2013.01) [A61K 51/0459 (2013.01); A61K 51/0476 (2013.01); A61K 51/0478 (2013.01); A61K 51/0497 (2013.01); A61K 51/08 (2013.01); A61B 2576/023 (2013.01)] | 16 Claims |
|
1. A method of evaluating risk of developing atrial fibrillation as a primary event comprising:
administering, to a subject, imaging agent 99mTc-RP805:
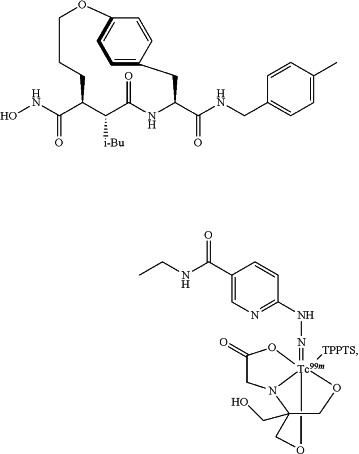 wherein TPPTS is 3,3′,3″-phosphanetriyltris(benzenesulfonic acid) trisodium salt, and
acquiring an image of the atria or the aortic valve region of the subject,
wherein the image indicates an aggregate level of MMP comprising MMP-2, MMP-9 and MMP-14 in the atria or the aortic valve region of the subject, and
wherein an aggregate level of MMP in the atria or the aortic valve region of the subject above control is indicative of an increased risk of developing atrial fibrillation.
|