| CPC A61K 31/704 (2013.01) [A61K 9/0014 (2013.01); A61P 17/06 (2018.01)] | 6 Claims |
|
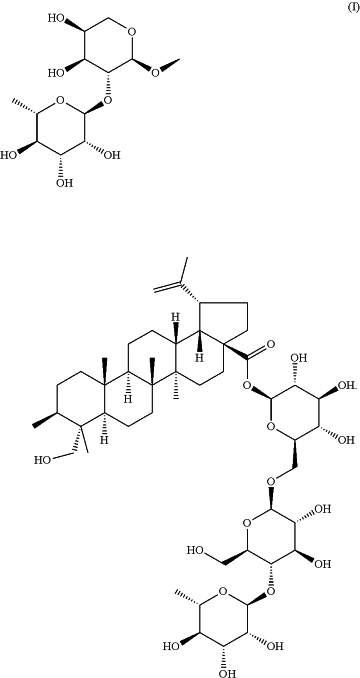
1. A method for treating psoriasis comprising a step of administering a therapeutic effective dosage of anemoside B4 with a structural formula (I) to a subject in need; wherein the therapeutic effective dosage of the anemoside B4 is 1-20 mg/kg per day;
 |