| CPC A61J 7/0076 (2013.01) [A61J 7/0427 (2015.05); G07F 11/62 (2013.01); G07F 17/0092 (2013.01); G08B 21/24 (2013.01); G16H 20/13 (2018.01); A61J 2200/30 (2013.01); A61J 2200/70 (2013.01)] | 14 Claims |
|
1. An apparatus, comprising:
a container configured to be closed with a cap; and
a cartridge for housing medication configured to be inserted within the container, the cartridge when completely inserted within the container allowing the closing of the container with the cap;
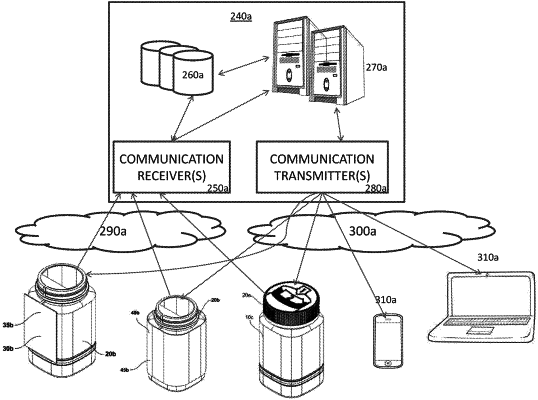
the cap including a cap sensor element configured to detect whether the container has not been activated within a predetermined period of time;
the container generating an alert based on the cap sensor detecting whether the container has not been activated within the predetermined period of time and whether a preset quantity of medication has not been withdrawn from the cartridge at a preset time, the alert being generated based on:
an adherence score characterizing a likelihood of adherence of a patient to a medication regimen associated with the medication, the adherence score being determined based on:
weighting a consumption of the medication at a first time period in accordance with a first percentage, the first time period corresponding to a designated time frame for the consumption of the medication,
weighting an additional consumption of the medication at a second time period in accordance with a second percentage, the second time period is different from and independent of the designated time frame for the consumption of the medication, and
wherein the first percentage is higher than the second percentage.
|