| CPC A61C 1/082 (2013.01) [A61B 6/03 (2013.01); A61B 18/06 (2013.01); A61B 18/082 (2013.01); A61B 18/148 (2013.01); A61B 18/1477 (2013.01); A61B 34/10 (2016.02); A61B 34/20 (2016.02); A61C 1/084 (2013.01); A61C 3/00 (2013.01); A61C 3/14 (2013.01); A61C 5/42 (2017.02); A61C 8/0089 (2013.01); A61C 13/0004 (2013.01); A61B 2017/00115 (2013.01); A61B 2018/00321 (2013.01); A61B 2018/00565 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00613 (2013.01); A61B 2018/00994 (2013.01); A61B 2018/0293 (2013.01); A61B 2018/1869 (2013.01); A61B 2018/2005 (2013.01); A61B 2034/108 (2016.02); A61B 2090/036 (2016.02); A61C 9/004 (2013.01); A61C 9/0006 (2013.01); A61N 2007/025 (2013.01); Y10T 29/49826 (2015.01)] | 15 Claims |
|
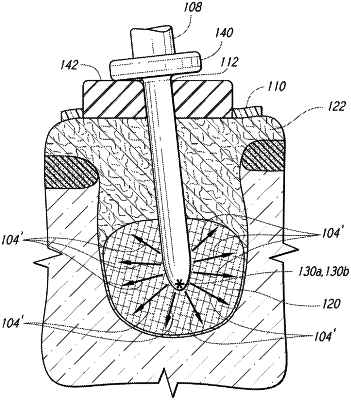
1. An ablation method for ablating tissue using a virtual stent, said method comprising the steps of:
(a) providing said virtual stent and a movement sensored ablation probe tip, said movement sensored ablation probe tip having an insertion end and a connection end, said movement sensored ablation probe tip having a center of ablation;
(b) providing at least one volume scan;
(c) providing at least one virtual surgical guide angle, said at least one virtual surgical guide angle providing angle guidance to guide said movement sensored ablation probe tip at a pre-defined angle; and
(d) providing at least one virtual stop, said at least one virtual stop providing stop information to limit the depth of said movement sensored ablation probe tip to a pre-defined depth;
(e) introducing said movement sensored ablation probe tip to said tissue;
(f) using said at least one virtual surgical guide angle and said at least one virtual stop, guiding said movement sensored ablation probe tip towards the position where said center of ablation is substantially within said tissue; and
(g) using ablation means, ablating said tissue when said center of ablation is within said tissue.
|