| CPC A61B 5/4052 (2013.01) [A61B 5/24 (2021.01); A61B 5/242 (2021.01); A61B 5/6857 (2013.01); A61B 18/1492 (2013.01); G01R 33/032 (2013.01); A61B 18/18 (2013.01); A61B 18/1815 (2013.01); A61B 2017/320069 (2017.08); A61B 2018/0022 (2013.01); A61B 2018/00267 (2013.01); A61B 2018/00434 (2013.01); A61B 2018/00511 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00636 (2013.01); A61B 2018/00642 (2013.01); A61B 2018/0212 (2013.01); A61B 2018/1435 (2013.01); A61B 2018/1807 (2013.01); A61B 2090/376 (2016.02); A61B 2090/378 (2016.02); A61B 2090/3735 (2016.02); G01R 33/26 (2013.01)] | 20 Claims |
|
1. A system comprising:
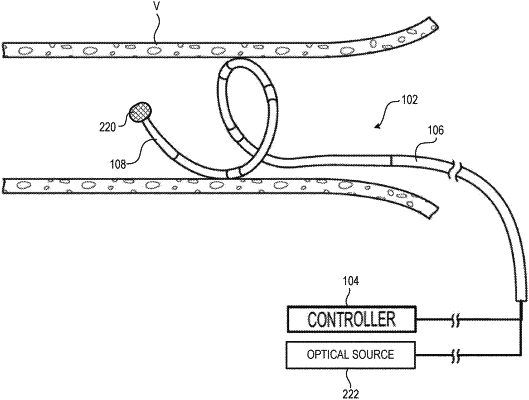
an optical magnetic sensor configured to generate a signal indicative of a measurement of a magnetic field generated by a target nerve within a patient; and
a controller configured to:
determine a baseline measurement of the magnetic field generated by the target nerve based on the signal generated by optical magnetic sensor before delivery of neuromodulation treatment to a target site within the patient, wherein the baseline measurement includes at least one of a magnitude of the magnetic field, a distance from the optical magnetic sensor to the target nerve, a magnitude of an impulse of the target nerve or a temporal extent of the impulse of the target nerve;
after the neuromodulation treatment is delivered at the target site, determine a post-neuromodulation measurement of the magnetic field generated by the target nerve based on the signal generated by the optical magnetic sensor, wherein the post-neuromodulation measurement includes the at least one of the magnitude of the magnetic field, the distance from the optical magnetic sensor to the target nerve, the magnitude of the impulse of the target nerve or the temporal extent of the impulse of the target nerve;
compare the baseline measurement to the post-neuromodulation measurement; and
based on the comparison of the baseline measurement to the post-neuromodulation measurement, determine a percentage that the target nerve was ablated by the delivered neuromodulation treatment.
|