| CPC C07H 19/12 (2013.01) [A61P 31/14 (2018.01); A61P 31/16 (2018.01)] | 18 Claims |
|
1. A compound having a structure represented by a formula:
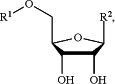 wherein R1 is selected from —C(O)R10, —C(O)CH(R11)NH2, and —P(O)(OAr1)NHCH(R12)CO2R13;
wherein R10, when present, is selected from C1-C20 alkyl and C2-C20 alkenyl;
wherein R11, when present, is a side chain of an amino acid selected from arginine, histidine, lysine, aspartic acid, glutamic acid, serine, threonine, asparagine, glutamine, cysteine, glycine, proline, alanine, valine, isoleucine, leucine, methionine, phenylalanine, tyrosine, and tryptophan;
wherein R12, when present, is selected from C1-C6 alkyl and C3-C6 cycloalkyl;
wherein R13, when present, is selected from C1-C8 alkyl, C3-C8 cycloalkyl, Ar2, and —CH2Ar2;
wherein Ar2, when present, is selected from C6-C14 aryl and C2-C10 heteroaryl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —CN, —NH2, —OH, —NO2, C1-C4 alkyl, C2-C4 alkenyl, C1-C4 haloalkyl, C1-C4 cyanoalkyl, C1-C4 hydroxyalkyl, C1-C4 haloalkoxy, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, and C1-C4 aminoalkyl;
wherein each of R14a and R14b, when present, is independently C1-C8 alkyl; and
wherein Ar1, when present, is selected from C6-C14 aryl and C2-C10 heteroaryl, and is substituted with 0, 1, 2, or 3 groups independently selected from halog'en, —CN, —NH2, —OH, —NO2, C1-C4 alkyl, C2-C4 alkenyl, C1-C4 haloalkyl, C1-C4 cyanoalkyl, C1-C4 hydroxyalkyl, C1-C4 haloalkoxy, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, and C1-C4 aminoalkyl; and
wherein R2 is a structure represented by a formula selected from:
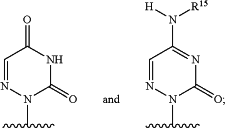 and
wherein R15, when present, is selected from hydrogen, —C(O)(C1-C20 alkyl), —C(O)(C3-C6 cycloalkyl), and —C(O)(C2-C20 alkenyl),
provided that when R2 is
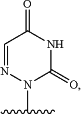 then R1 is —C(O)CH(R11)NH2 or —P(O)(OAr1)NHCH(R12)CO2R13,
or a pharmaceutically acceptable salt thereof.
|