| CPC C07D 487/04 (2013.01) [A61K 45/06 (2013.01); C07D 519/00 (2013.01)] | 12 Claims |
|
1. A compound having the chemical structure:
PTM-L-CLM,
or a pharmaceutically acceptable salt thereof,
wherein:
(a) the CLM is represented by the chemical structure:
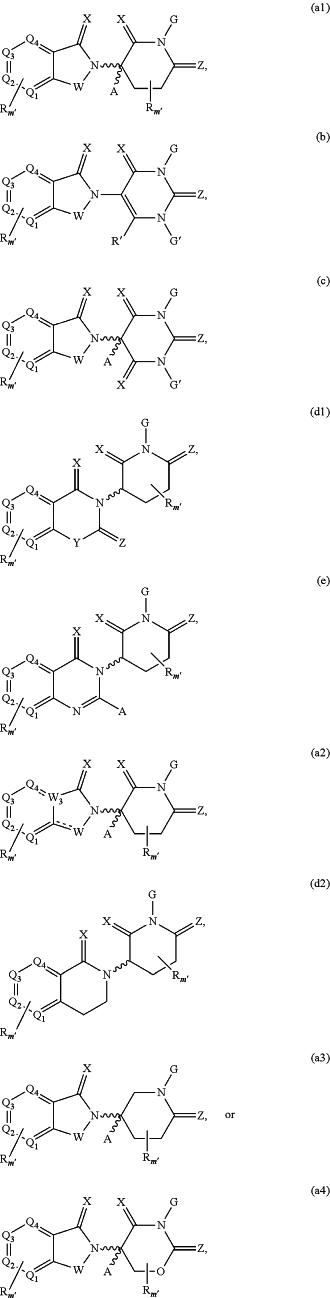 wherein:
W is selected from CH2, O, CHR, C═O, SO2, NH, N, optionally substituted cyclopropyl group, optionally substituted cyclobutyl group, and N-alkyl;
W3 is C or N;
each X is independently selected from absent, O, S, and CH2,
Y is selected from CH2, —C═CR′, NH, N-alkyl, N-aryl, N-heteroaryl, N-cycloalkyl, N-heterocyclyl, O, and S;
Z is selected from absent, O, S, and CH2;
G and G′ are independently selected from H, unsubstituted or substituted linear or branched alkyl, OH, R′OCOOR, R′OCONRR″, CH2-heterocyclyl optionally substituted with R′, and benzyl optionally substituted with R′;
Q1, Q2, Q3, and Q4 represent C or N substituted with a group independently selected from H, R, N, and N-oxide;
A is independently selected from H, unsubstituted or substituted linear or branched alkyl, cycloalkyl, —Cl, and —F;
m′ is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
R is a bond, H, —CONR′R″, —C(═O)R′, —OR′, —NR′R″, —SR′, —SO2R′, —SO2NR′R″, —CR′R″—, —CR′NR′R″—, (—CR′O)n′R″, optionally substituted heterocyclyl, aryl, optionally substituted alkyl-aryl, heteroaryl, unsubstituted or substituted linear or branched alkyl, optionally substituted alkoxyl group, optionally substituted heterocyclyl, —P(O)(OR′)R″, —P(O)R′R″, —OP(O)(OR′)R″, —OP(O)R′R″, —Cl, —F, —Br, —I, —CF3, —CN, —NR′SO2NR′R″, —NR′CONR′R″, —CONR′COR″, —NR′C(═N—CN)NR′R″, —C(═N—CN)NR′R″, —NR′C(═N—CN)R″, —NR′C(═C—NO2)NR′R″, —SO2NR′COR″, —NO2, —CO2R′, —C(C═N—OR′)R″, —CR′═CR′R″, —CCR′, —S(C═O)(C═N—R′)R″, —SF5 and —OCF3, wherein at least one R or W is modified to be covalently joined to the PTM, or the CLM;
R′ and R″ are independently selected from a bond, H, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted heteroaryl, optionally substituted heterocyclic, and optionally substituted heterocyclyl;
n′ is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
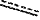 represents a single bond or a double bond; and represents a single bond or a double bond; and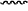 represents a bond that may be stereospecific or non-stereospecific; represents a bond that may be stereospecific or non-stereospecific;(b) the PTM has a chemical structure selected from:
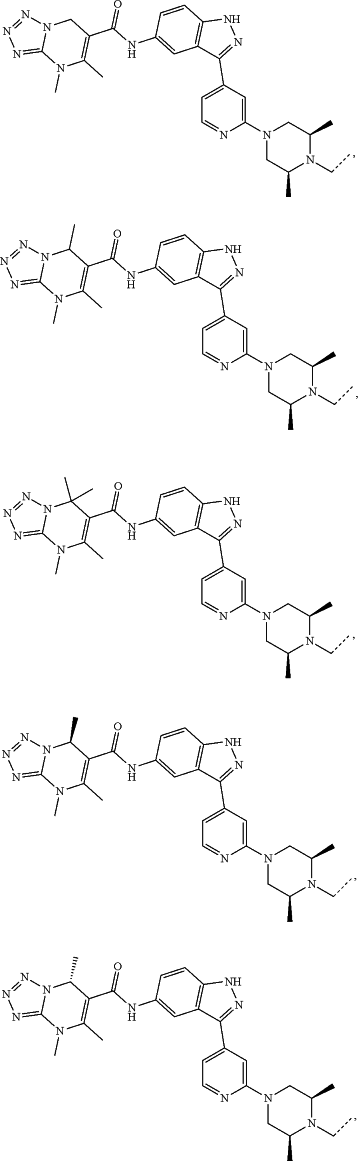 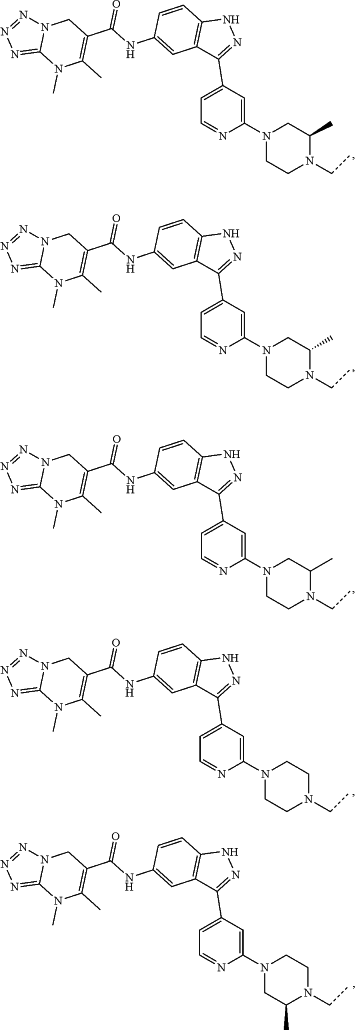 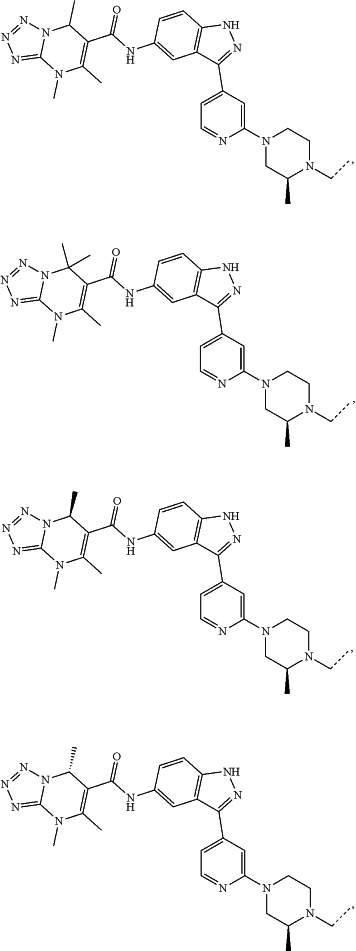 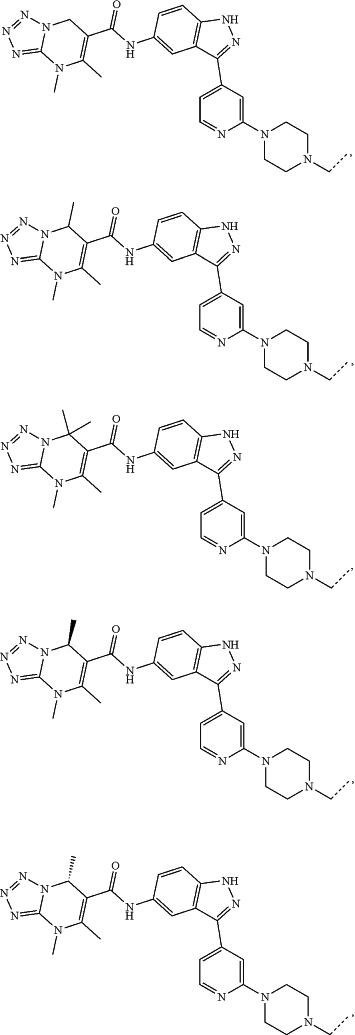 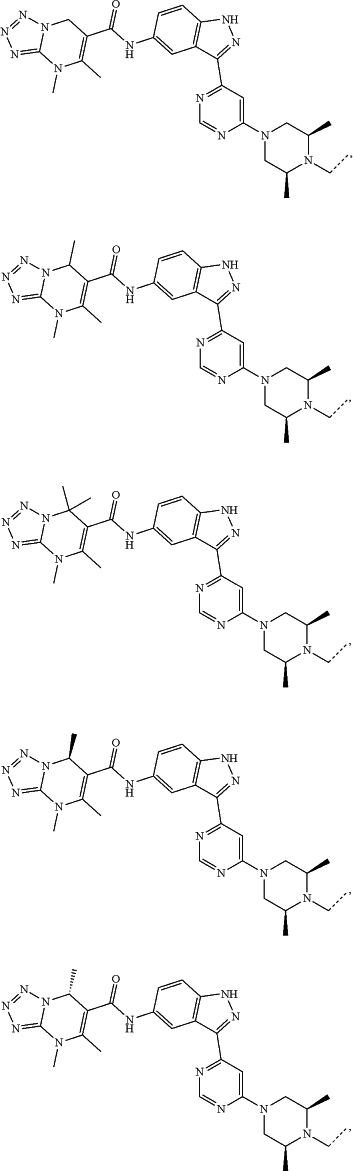 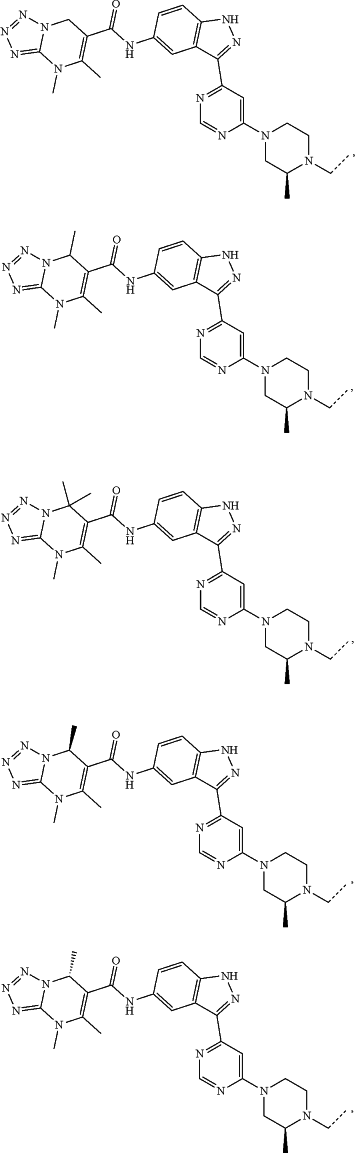 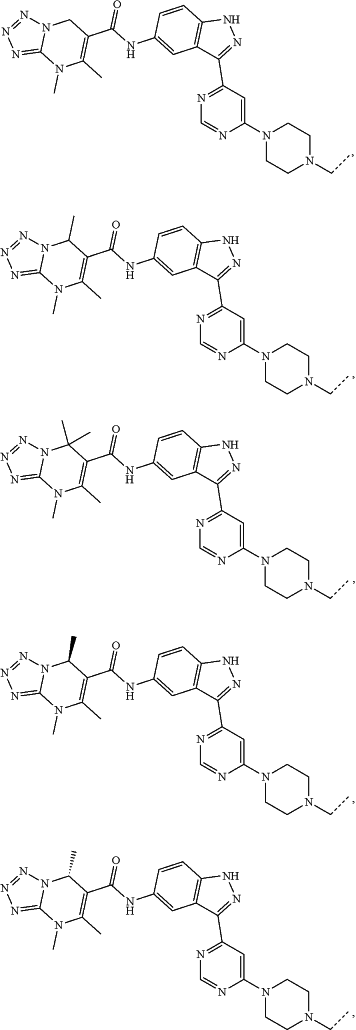 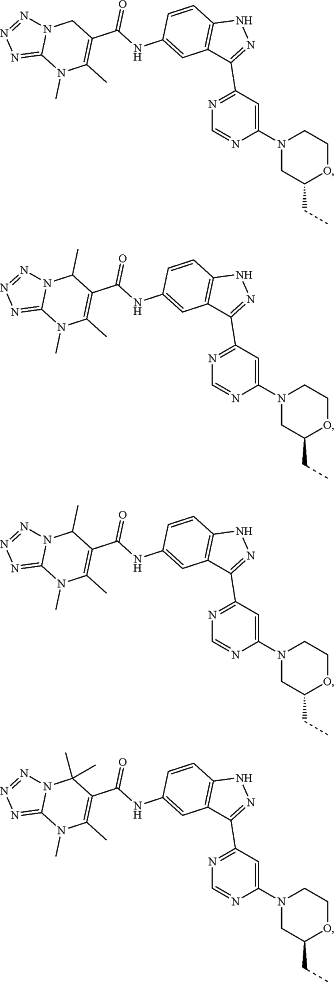 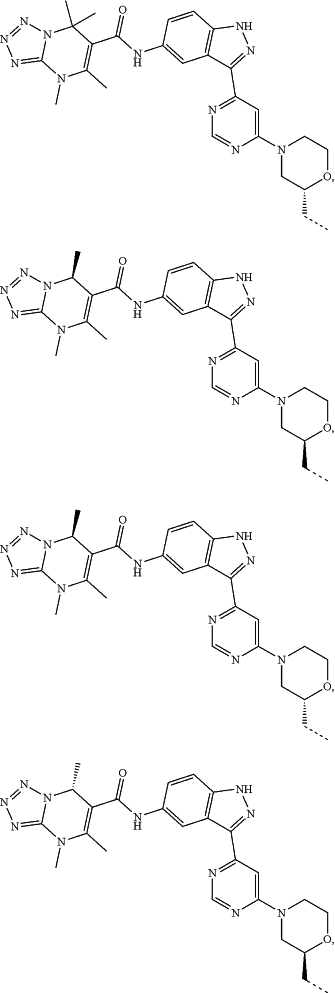 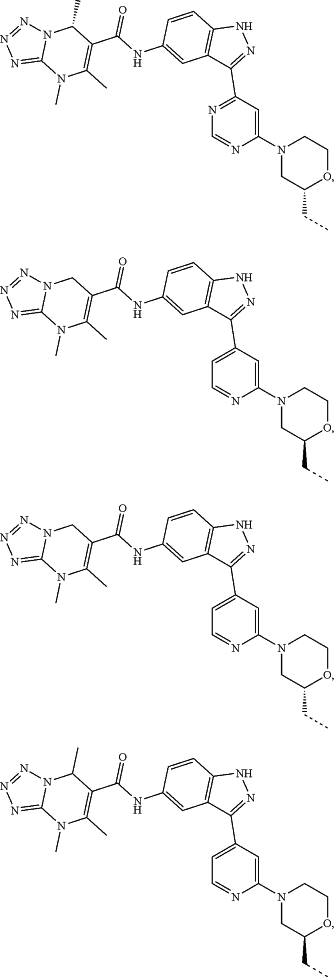 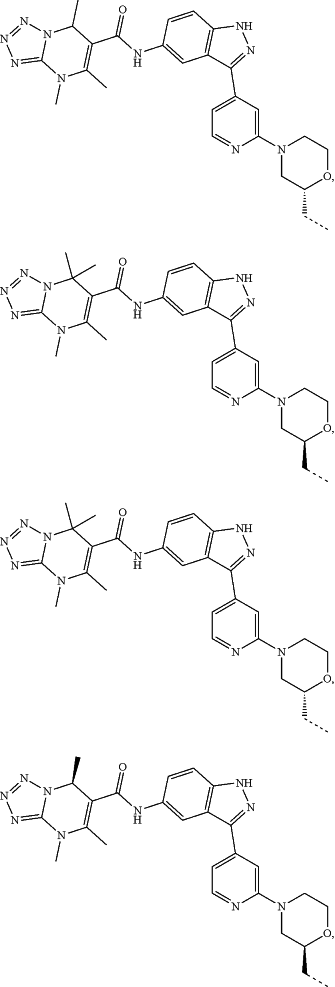 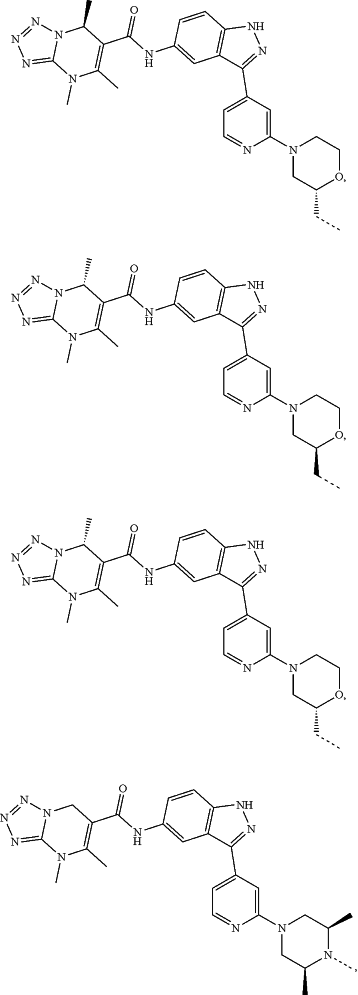 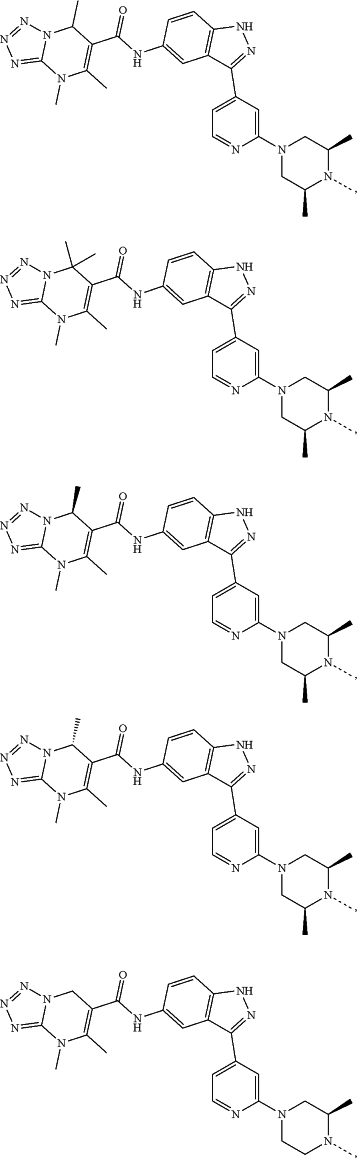 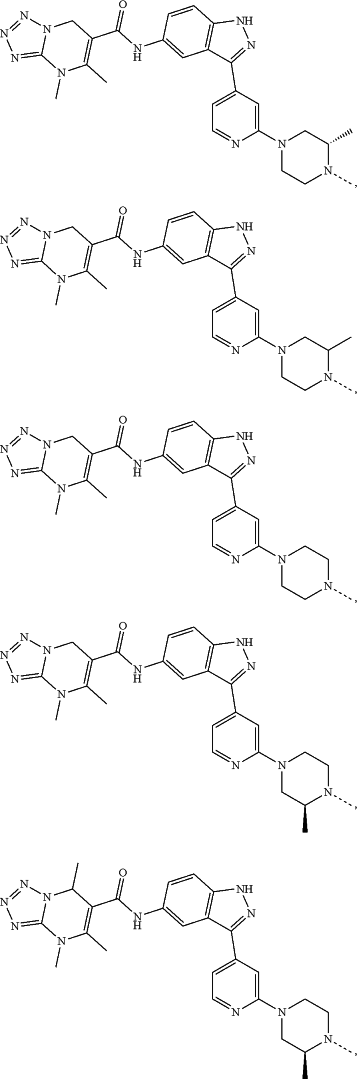 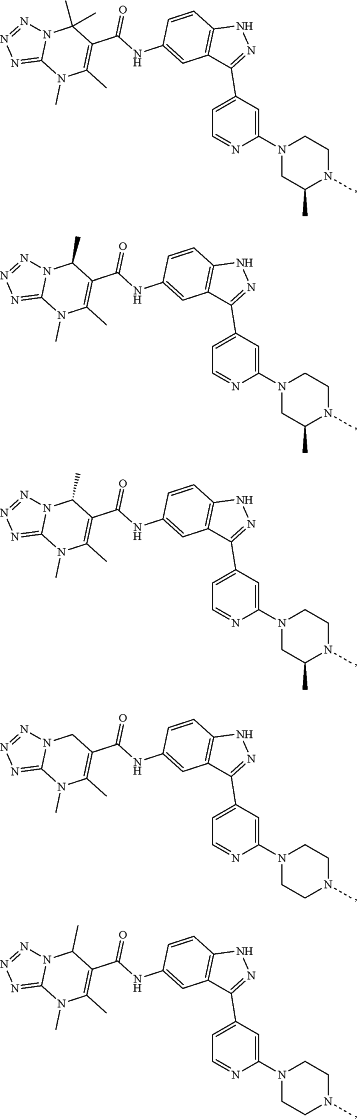 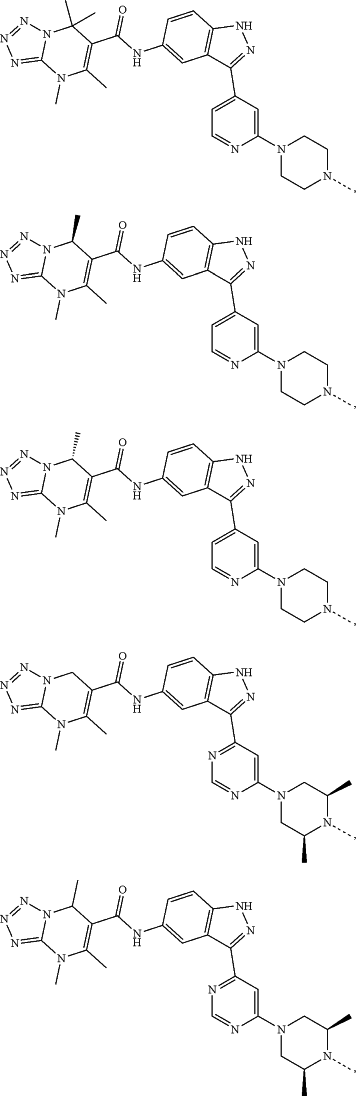 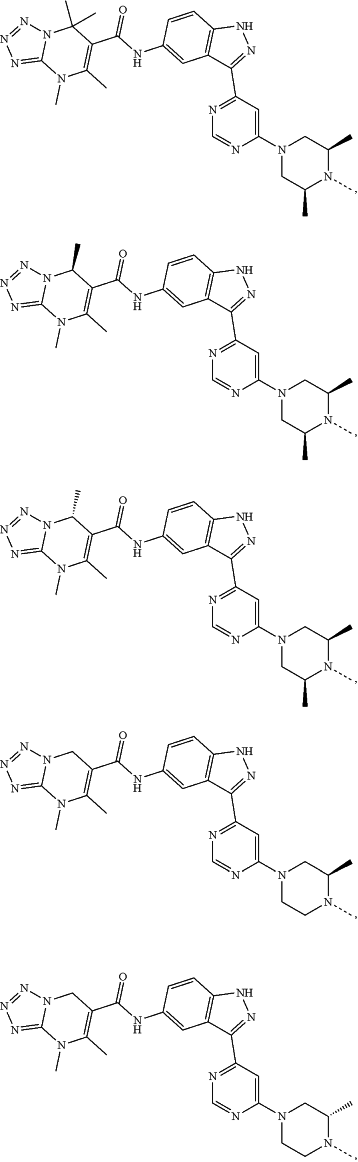 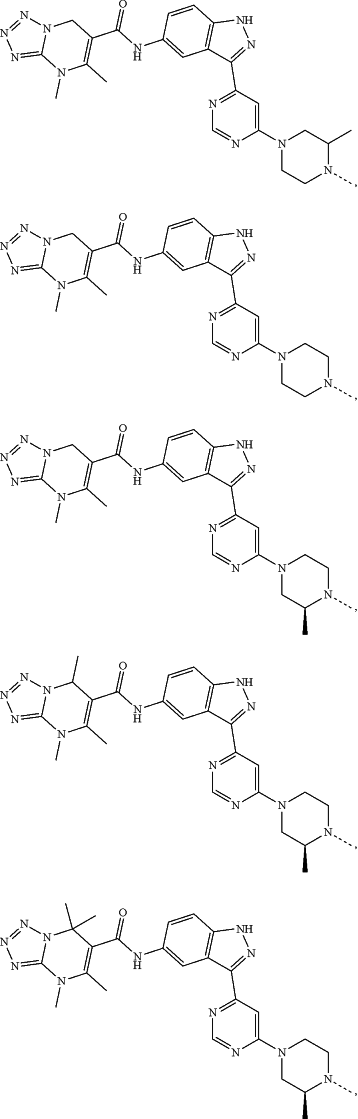 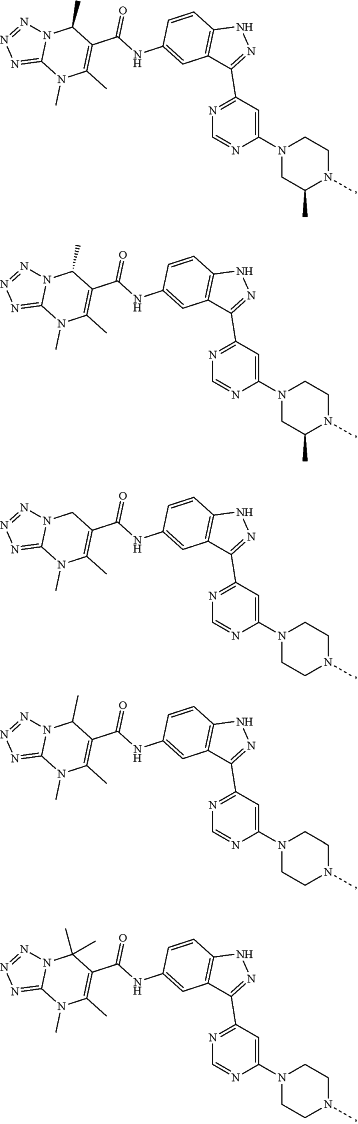 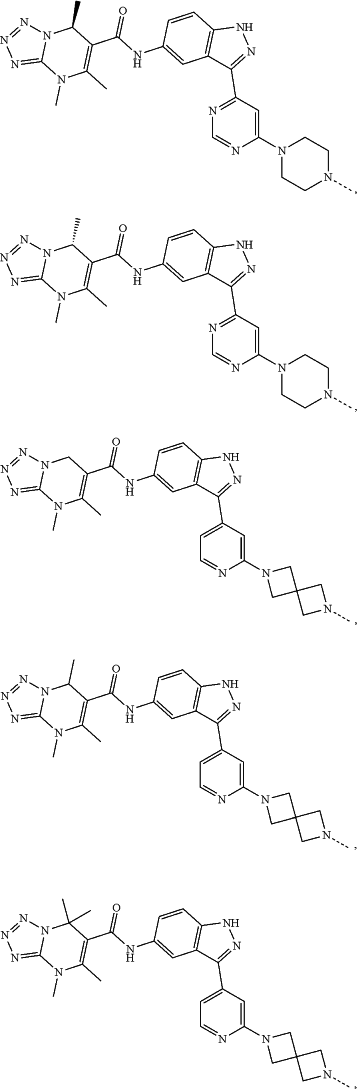 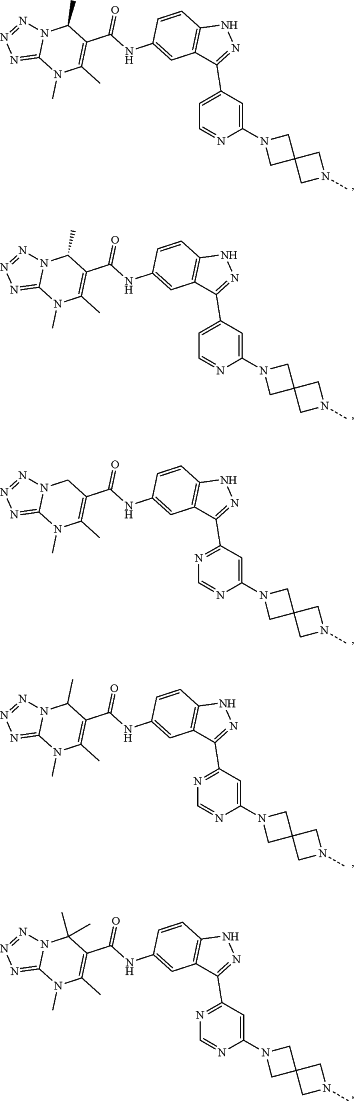 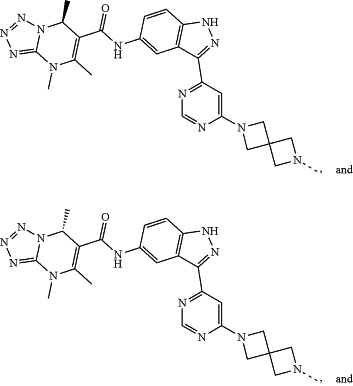 wherein the
 of the PTM indicates the site of attachment with a chemical linking group; and of the PTM indicates the site of attachment with a chemical linking group; and(c) the L is a bond or is a chemical linking group that covalently couples the CLM to the PTM and is selected from:
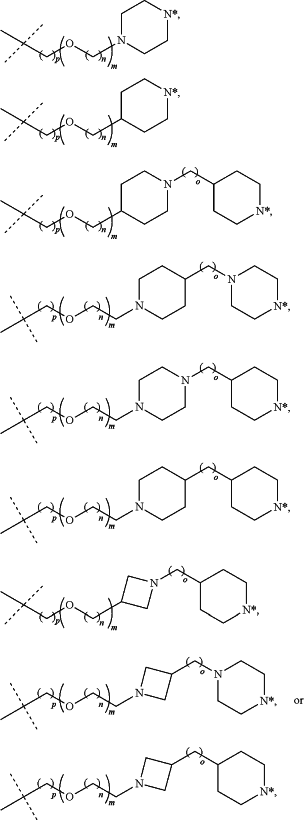 wherein:
N* is a nitrogen atom that is covalently linked to the CLM or the PTM, or that is shared with the CLM or the PTM;
each m, n, o, and p is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20; and
the chemical linker group is optionally substituted with 1, 2, 3, or 4 substitutions independently selected from halogen and C1-4 alkyl.
|