| CPC C07D 471/04 (2013.01) [A61P 15/08 (2018.01)] | 21 Claims |
|
1. A method of treating polycystic ovary syndrome with functional adrenal hyperandrogenism (PCOS-FAH) in a subject in need thereof, comprising administering a CRF1 antagonist or pharmaceutically acceptable salt thereof;
wherein said CRF1 antagonist is a compound of Formula (I):
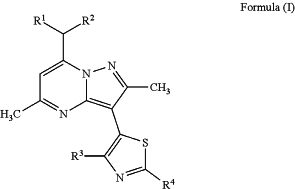 or a pharmaceutically acceptable salt thereof, wherein:
R1 and R2 are independently ethyl or n-propyl;
R3 is hydrogen, F, Cl, Br, methyl, trifluoromethyl, or methoxy;
R4 is hydrogen, Br, —NRaRb, methoxymethyl, n-butyl, acetamido, pyridin-4-yl,
morpholin-4-yl,
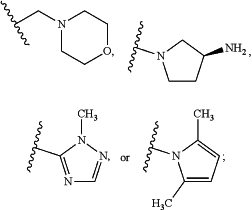 Ra and Rb are independently hydrogen, C1-C3alkyl, —CH2CH2NH2, —CH2CH2NHC(O)OC(CH3)3, or —CH2CH2NHCH2CH2CH3.
|