| CPC C07D 307/93 (2013.01) [H10K 85/615 (2023.02); H10K 85/636 (2023.02); H10K 85/655 (2023.02); H10K 85/6574 (2023.02); H10K 50/15 (2023.02); H10K 50/16 (2023.02); H10K 50/171 (2023.02); H10K 50/18 (2023.02)] | 18 Claims |
|
1. An amine compound represented by the following Chemical Formula A or Chemical Formula B:
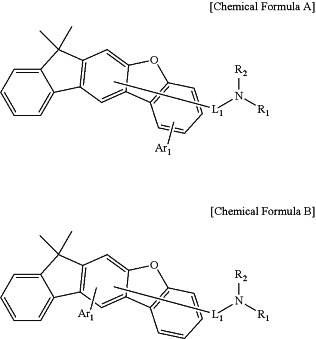 wherein,
Ar1 is one selected from a substituted or unsubstituted alkyl of 1 to 30 carbon atoms, a substituted or unsubstituted haloalkyl of 1 to 30 carbon atoms, a substituted or unsubstituted aryl of 6 to 50 carbon atoms, a substituted or unsubstituted cycloalkyl of 3 to 30 carbon atoms, a substituted or unsubstituted heteroaryl of 2 to 50 carbon atoms, a substituted or unsubstituted alkylsilyl of 1 to 30 carbon atoms, and a substituted or unsubstituted arylsilyl of 5 to 30 carbon atoms,
L1 is one selected from a single bond, a substituted or unsubstituted arylene of 6 to 30 carbon atoms, and a substituted or unsubstituted heteroarylene of 1 to 30 carbon atoms,
R1 and R2, which are same or different, are each independently one selected from a substituted or unsubstituted alkyl of 1 to 30 carbon atoms, a substituted or unsubstituted haloalkyl of 1 to 30 carbon atoms, a substituted or unsubstituted aryl of 6 to 50 carbon atoms, a substituted or unsubstituted arylalkyl of 7 to 50 carbon atoms, a substituted or unsubstituted cycloalkyl of 3 to 30 carbon atoms, a substituted or unsubstituted heteroaryl of 2 to 50 carbon atoms, a substituted or unsubstituted alkoxy of 1 to 30 carbon atoms, a substituted or unsubstituted aryloxy of 6 to 30 carbon atoms, a substituted or unsubstituted alkylthioxy of 1 to 30 carbon atoms, a substituted or unsubstituted arylthioxy of 5 to 30 carbon atoms, a substituted or unsubstituted alkylamine of 1 to 30 carbon atoms, a substituted or unsubstituted arylamine of 5 to 30 carbon atoms, a substituted or unsubstituted alkylsilyl of 1 to 30 carbon atoms, a substituted or unsubstituted arylsilyl of 5 to 30 carbon atoms, a cyano, a nitro, and a halogen,
R1 and R2 can be connected to each other to form a ring, and
each of the carbon atoms on the aromatic rings of the indenodibenzofuran fuse ring moiety
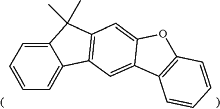 in Chemical Formulas A and B can be bound with a hydrogen or deuterium, except for the carbon atoms bound with Ar1 or L1,
wherein the term “substituted” in the expression “substituted or unsubstituted” means having at least one substituent selected from the group consisting of a deuterium, a cyano, a halogen, a hydroxy, a nitro, an alkyl of 1 to 24 carbon atoms, a cycloalkyl of 3 to 24 carbon atoms, a halogenated alkyl of 1 to 24 carbon atoms, an alkenyl of 2 to 24 carbon atoms, an alkynyl of 2 to 24 carbon atoms, a heteroalkyl of 1 to 24 carbon atoms, an aryl of 6 to 24 carbon atoms, an arylalkyl of 7 to 24 carbon atoms, an alkylaryl of 7 to 24 carbon atoms, a heteroaryl of 2 to 24 carbon atoms, a heteroarylalkyl of 2 to 24 carbon atoms, an alkoxy of 1 to 24 carbon atoms, an alkylamino of 1 to 24 carbon atoms, a diarylamino of 12 to 24 carbon atoms, a diheteroarylamino of 2 to 24 carbon atoms, an aryl(heteroaryl)amino of 7 to 24 carbon atoms, an alkylsilyl of 1 to carbon atoms, an arylsilyl of 6 to 24 carbon atoms, an aryloxy of 6 to 24 carbon atoms, and an arylthionyl of 6 to 24 carbon atoms.
|