| CPC C07C 217/22 (2013.01) [C07D 295/13 (2013.01); A61K 45/06 (2013.01)] | 8 Claims |
|
1. A compound according to Formula 1 below:
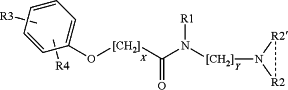 wherein
X is 1-3;
Y is 1-3;
R1 is methyl, ethyl, propyl, butyl, or haloalkyl;
R2 and R2′ are independently selected from methyl, propyl, butyl, alkoxy, haloalkyl, and hydrogen or R2 and R2′ may be fused into a heterocyclic ring with 5 or 6 members;
R3 is methyl, ethyl, alkoxy, carboxylic acid, or haloalkyl; and
R4 is hydrogen, hydroxyl or halogen,
or a pharmaceutically acceptable salt, addition compound or pro-drug thereof.
|