| CPC C01C 1/26 (2013.01) [B01D 9/00 (2013.01); C02F 9/00 (2013.01); C05C 11/00 (2013.01); B01D 9/0004 (2013.01); B01D 2009/0086 (2013.01); C02F 1/02 (2013.01); C02F 1/20 (2013.01); C02F 1/22 (2013.01); C02F 1/441 (2013.01); C02F 1/444 (2013.01); C02F 1/447 (2013.01); C02F 3/28 (2013.01); C02F 2001/5218 (2013.01); C02F 2101/16 (2013.01); C02F 2103/20 (2013.01); C02F 2209/02 (2013.01); C02F 2303/24 (2013.01)] | 25 Claims |
|
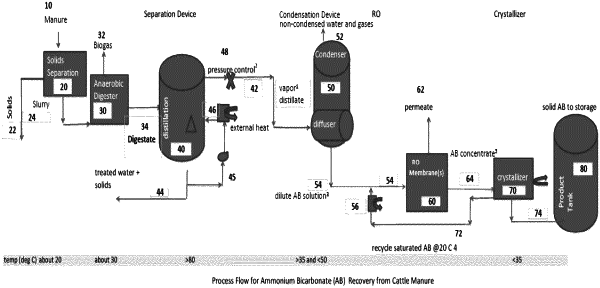
1. A process to treat waste containing ammonia nitrogen comprising:
treating waste at a temperature of at least 80 degrees Celsius to create a gas and, without the addition of chemicals that are not inherently present in the waste, converting substantially all ammonia in the waste to gaseous form in the gas, said gas also containing carbon dioxide and water vapor;
treating said gas containing gaseous ammonia, carbon dioxide and water vapor, using partial condensation, or repeated partial condensation, to concentrate the ammonia and carbon dioxide; all without the use of chemical(s) that are not inherently present in the waste, and remove from said gas a significant amount of water vapor in liquid condensate form, causing formation of a concentrated gas containing higher concentrations of ammonia gas and carbon dioxide gas than before condensation;
treating said concentrated gas using absorption or adsorption to form concentrated ammonium carbonate and ammonium bicarbonate;
crystallizing the concentrated ammonium carbonate and ammonium bicarbonate at a temperature of less than about 35 degrees Celsius to form solid ammonium bicarbonate and ammonium carbonate.
|