| CPC A61K 9/0024 (2013.01) [A61F 2/022 (2013.01); A61K 31/337 (2013.01); A61K 31/397 (2013.01); A61K 31/436 (2013.01); A61K 31/65 (2013.01); A61K 31/7036 (2013.01); A61K 35/28 (2013.01); A61K 38/14 (2013.01); A61K 38/1866 (2013.01); A61K 38/22 (2013.01); A61K 39/395 (2013.01); A61K 47/02 (2013.01); A61M 39/0247 (2013.01); A61M 2039/027 (2013.01); A61M 2039/0261 (2013.01); A61M 2039/0276 (2013.01); A61M 2205/04 (2013.01)] | 18 Claims |
|
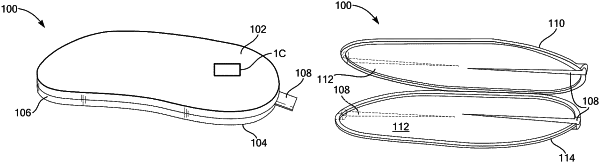
1. An implantable medical device, comprising:
a first portion having a first nanoscale through-porous structure, the first nanoscale through-porous structure having a surface treatment pattern configured to increase a surface area of exposure of the first nanoscale through-porous structure to an exterior of the implantable medical device; 121
a second portion having a second nanoscale through porous structure opposite the first portion; and
the first portion and the second portion being engaged to an implantable spacer ring.
|