|
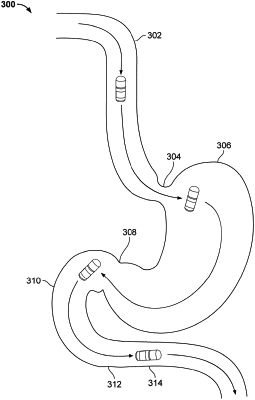
1. A method of treating a disease of the gastrointestinal (GI) tract in a subject, the method comprising orally administering to the subject a pharmaceutical composition comprising a therapeutically effective amount of a Janus Kinase (JAK) inhibitor and not comprising any additional therapeutic agent, and delivering the therapeutically effective amount of the JAK inhibitor at a desired location in the GI tract of the subject, wherein the therapeutically effective amount of the JAK inhibitor is less than an amount that is effective when the JAK inhibitor is administered systemically, wherein the JAK inhibitor is selected from the group consisting of tofacitinib, filgotinib, TD-1473, ruxolitinib, cerdulatinib, momelotinib, oclacitinib, upadacitinib, lestaurtinib, decernotinib, pacritinib, cucurbitacin I, PF-06700841, PF-06651600, PF-04965842, and generic equivalents thereof, and wherein the desired location is proximate to one or more sites of disease.
|