| CPC A61K 49/0052 (2013.01) [A61K 9/0019 (2013.01); A61K 49/0032 (2013.01); A61K 49/0034 (2013.01); C09B 23/0025 (2013.01); C09B 23/0066 (2013.01); G01N 33/57434 (2013.01)] | 20 Claims |
|
1. A sterile, non-toxic pharmaceutical composition suitable for administration to a patient, comprising:
(a) a pharmaceutically acceptable excipient; and
(b) a unit dosage form of a compound having the formula:
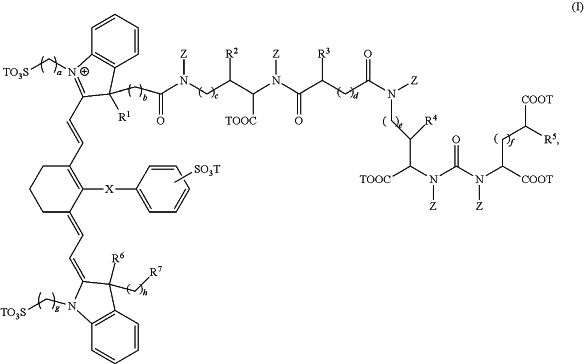 wherein:
R1, R2, R3, R4, R5, R6, and R7 are each independently selected from the group consisting of hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, and tert-butyl;
X is selected from the group consisting of a single bond, —O—, and —S—;
subscripts a, b, c, d, e, f, g, and h are each independently an integer selected from 1 to 6;
each T is independently selected from the group consisting of a metal ion, H, and a negative charge; and
each Z is independently selected from the group consisting of hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-pentyl, branched pentyl, n-hexyl, and branched hexyl, and
wherein the unit dosage form delivers to the patient between 0.01 and 8 mg/kg of the compound of formula (I); and
wherein the pharmaceutical composition is adapted for visualization of tissue expressing PSMA under illumination at wavelengths ranging from 700 to 1400 nm.
|