| CPC A61K 31/74 (2013.01) [A61K 38/02 (2013.01); A61K 47/64 (2017.08); A61K 47/65 (2017.08)] | 12 Claims |
|
1. A Ligand Drug Conjugate compound represented by Formula 1:
L-[LU-D′]p′ (1)
or a salt thereof, wherein
L is a Ligand Unit;
subscript p′ is an integer ranging from 1 to 24:
each -LU-D′ is a drug-linker moiety of Formula 1A:
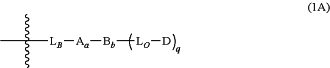 or a salt thereof,
wherein the wavy line indicates covalent attachment to L;
LB is a ligand covalent binding moiety;
A is a first optional Stretcher Unit;
subscript a is 0 or 1, indicating the absence or presence of A, respectively;
B is an optional Branching Unit;
subscript b is 0 or 1, indicating the absence or presence of B, respectively;
LO is an optional secondary linker moiety;
subscript q is an integer ranging from 1 to 4;
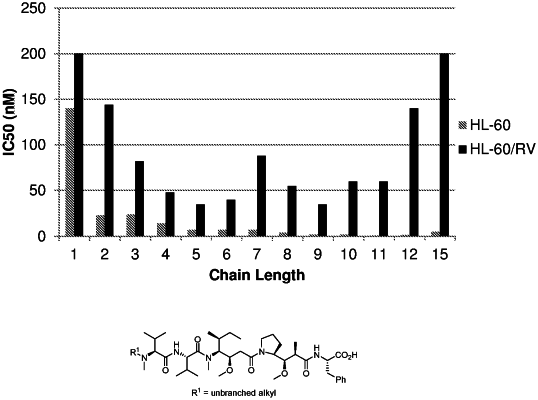
D is a hydrophobic auristatin F compound represented by the structure of:
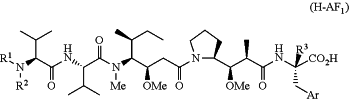 or a salt thereof, wherein
the hydrophobic auristatin F compound is conjugated to the remainder of the drug-linker moiety through its C-terminal component's carboxylic acid carbon atom:
Ar is phenyl, thienyl, 1-napthyl, 2-napthyl, or benzo[b]thiophen-3-yl;
R3 is independently selected from the group consisting of hydrogen and C1-C2 alkyl:
R1 is C1-C9 alkyl, or
R1 is (C3-C6 carbocyclyl)-alkylene- of up to 9 total carbon atoms, or
R1 is —(C2-C6 alkylene)-X—R4, wherein X is an amide or carbamate functional group and R4 is C1-C6 alkyl; and
R2 is C1-C2 alkyl,
with the proviso that the total number of carbon atoms in R2 and the (carbocyclyl)alkyl(ene) moiety of R1 is between 4 and 10, and R1 and R2 are not each methyl,
wherein, when present, LO has the formula of:
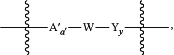 wherein the wavy line adjacent to Y indicates the site of covalent attachment to the hydrophobic auristatin F compound, and the wavy line adjacent to A′ indicates the site of covalent attachment to the remainder of the drug linker moiety;
A′ is a second optional Stretcher Unit,
subscript a′ is 0 or 1, indicating the absence or presence of A′, respectively;
W is a peptide Cleavable Unit;
Y is a peptide Spacer Unit; and
subscript y is 0 or 1, indicating the absence or presence of Y, respectively.
|