| CPC A61K 31/44 (2013.01) [A61K 9/0053 (2013.01); A61K 31/4412 (2013.01); A61K 33/26 (2013.01); A61K 38/1816 (2013.01)] | 30 Claims |
|
1. A method for treating anemia secondary to or associated with chronic kidney disease, comprising orally administering to a patient having said anemia an effective amount of a compound which is {[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino}acetic acid having the structure,
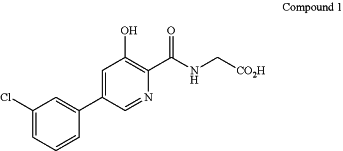 or a pharmaceutically acceptable salt thereof, wherein a daily dose comprises 150±30 mg, 300±30 mg, 450±30 mg, 600±30 mg, or 750±30 mg of the compound.
|