| CPC A61F 9/0008 (2013.01) [A61M 37/0092 (2013.01); A61F 2250/0093 (2013.01)] | 18 Claims |
|
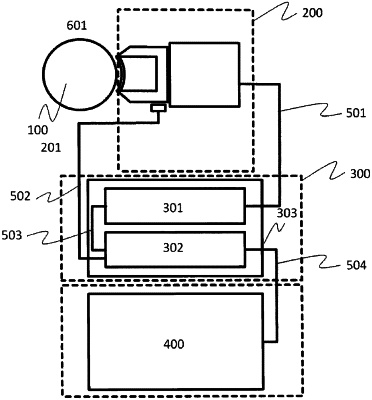
1. A drug applicator for a system for ultrasound-enhanced delivery of at least one drug to a target site in a intraocular space, the system comprising a signal generating unit operationally connected to an ultrasound transducer, and a controller,
wherein the drug applicator:
(a) comprises at least one space configured to hold the drug,
(b) is made from a low ultrasound attenuation material,
(c) is configured to provide low ultrasound loss coupling of the system to the sclera or cornea, and
(d) is configured to be mechanically coupled to the system and further configured to be coupled to the ultrasound transducer with low ultrasound loss; and
wherein the signal generating unit or the ultrasound transducer is configured to emit an ultrasound wave form in at least one application cycle, the application cycle comprising at least one ultrasound emitting event with a time duration TA and a waiting period with a time duration TW,
wherein, the controller obtains data from an information receiving or sending unit pertaining to the properties of the drug, and based on the properties of the drug controls a plurality of parameters pertaining to the application cycle comprising at least the time duration TA and time duration TW, such that (1) when the drug comprises molecules with a size of 70 kDa or less, controls the time duration TA to be between 30 s and 300 s and the drug applicator causes the drug to transit through the sclera during the time duration TA; and (2) when the drug comprises molecules with a size of more than 70 kDa, controls the time duration TW to be between 60 s and 600 s and the drug applicator causes the drug to transit through the sclera during the time duration TW.
|