| CPC A61F 2/442 (2013.01) [A61F 2/4455 (2013.01); A61F 2/4611 (2013.01); A61F 2/2846 (2013.01); A61F 2002/2835 (2013.01); A61F 2002/4629 (2013.01); A61F 2250/0018 (2013.01); A61F 2310/00023 (2013.01)] | 19 Claims |
|
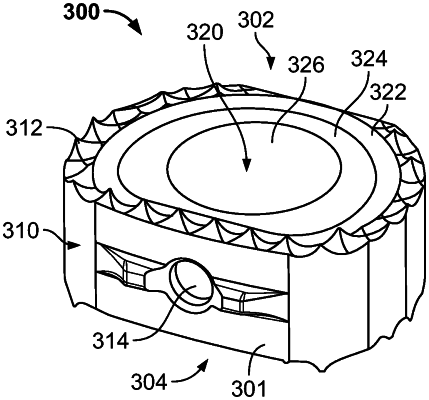
1. A spinal interbody device (IBD), comprising:
a solid structure; and
a porous body, including a plurality of sections of different porosity than an adjacent section, connected to the solid structure and being defined by a plurality of adjoining cells and pores situated between the cells.
|